New therapeutic options being currently investigated in advanced or metastatic colorectal cancer
Anti-EGFR antibody therapy in RAS and BRAF wild-type metastatic colorectal cancer
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, and it is the fourth most frequent cancer diagnosis [1].A current treatment option for RAS and BRAF wild-type (WT) metastatic colorectal cancer (mCRC) is the chemotherapy doublet (FOLFOX/FOLFIRI) with an anti-EGFR monoclonal antibody (cetuximab or panitumumab) [2, 3]. Early promising results have been reported in several trials that investigated the combination of intensified upfront chemotherapy regimens with an anti-EGFR agent, although high rates of gastrointestinal toxicities requiring dose modifications were observed [4, 5]. The VOLFI study (NCT01328171), a phase 2 randomized trial, demonstrated that the association of panitumumab with a modified schedule of FOLFOXIRI led to a higher objective response rate (ORR) than FOLFOXIRI alone (87% vs 61%, p=0.004) in previously untreated RAS-WT mCRC patients; however, no significant progression-free survival (PFS) difference was seen [6].
To assess the added value of an intensified first-line chemotherapy combined with panitumumab, a study was performed in a selected population of mCRC patients (RAS– and BRAF-WT) [6, 7]. The prospective, open-label, phase 3 trial TRIPLETE (NCT03231722) enrolled previously untreated patients with unresectable RAS– and BRAF-WT mCRC and randomized them to receive mFOLFOX6/pan (arm A) or mFOLFOXIRI (irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, L-leucovorin (LV) 200 mg/m2, 5-fluoruracil (5FU) 2,400 mg/m2 48 h infusion)/pan (arm B) up to twelve cycles, followed by 5FU/LV/pan until disease progression. Given an objective response rate (ORR) of 60% in arm A, an attempt was made to detect an increase of at least 15% in arm B. The primary endpoint in this trial was the ORR according to RECIST v1.1.
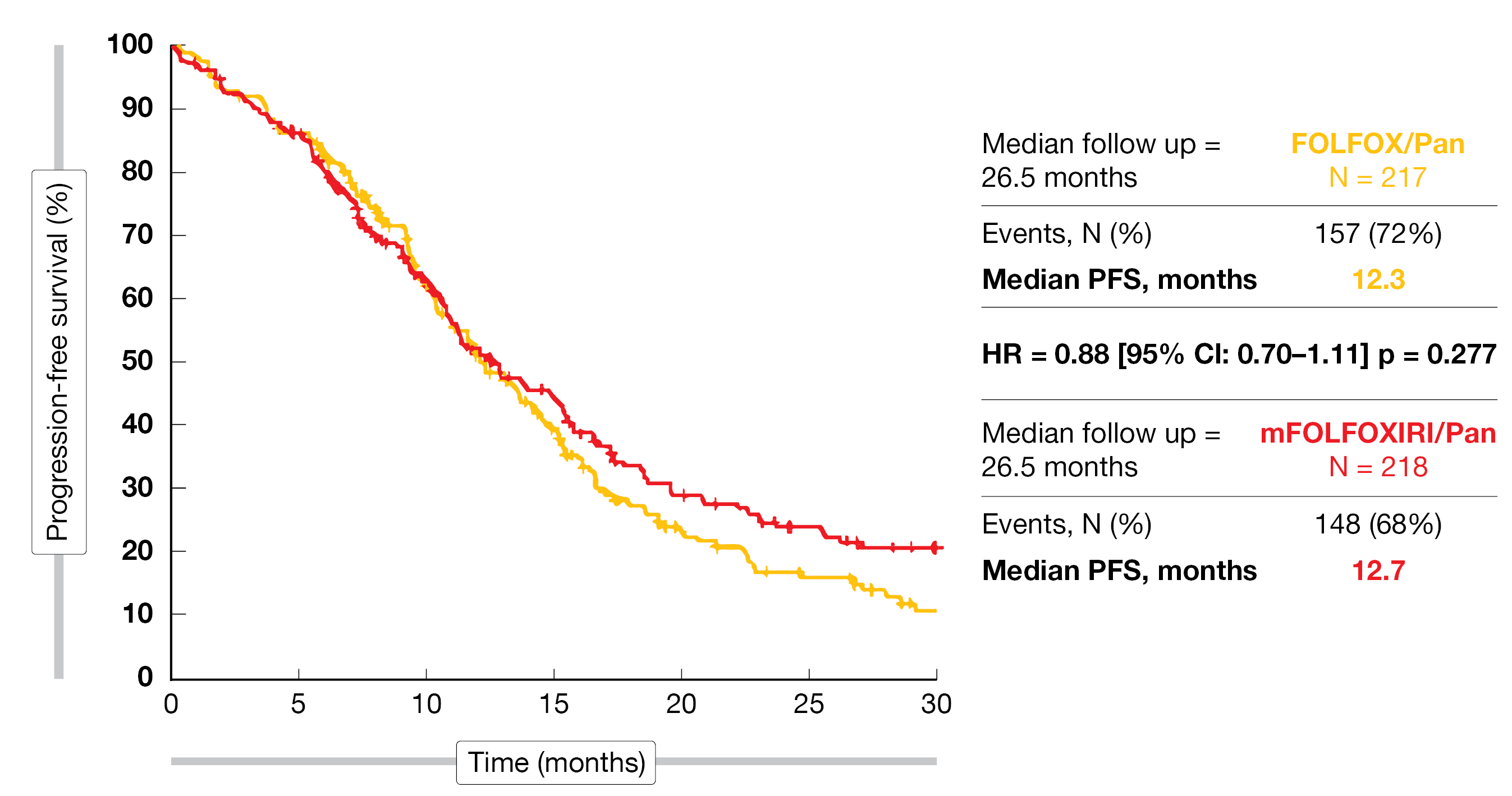
In total, 435 patients were enrolled in 67 Italian sites during the study period (Sept 2017-Sept 2021). Characteristics of the patients enrolled in this study were (arm A/B): median age 59/59 years, ECOG PS 0 80%/84%, synchronous metastases 88%/87%, prior adjuvant chemotherapy 2%/6%, resected primary tumor 43%/51%, liver-only disease 37%/39%. The primary endpoint (ORR) for this study was not met, as no significant difference was observed between both arms (76% vs 73%; OR, 0.87; 95% CI, 0.56-1.34; p=0.526). Considering the secondary endpoints, neither the R0 resection rate (29% vs 25%; OR, 0.81; 95% CI, 0.53-1.23; p=0.317), nor the median PFS (12.3 vs 12.7 months; HR, 0.88; 95% CI, 0.70-1.11; p=0.277), showed any benefit for one study arm above the other (Figure 1).
The main reported grade 3 and 4 adverse events (AEs) were skin rash (29%/19%, arm A/B), neutropenia (20%/32%), diarrhea (7%/23%) or stomatitis (7%/7%).
In RAS– and BRAF-WT mCRC patients, the intensification of the upfront chemotherapy backbone with mFOLFOXIRI was not associated with an improved response as compared to mFOLFOX6 when both regimens were combined with panitumumab.
Figure 1: Progression-free survival in both arms of the TRIPLETE trial.
Panitumumab plus mFOLFOX6 as 1L therapy in RAS-WT mCRC
The combination of an anti-EGFR or anti-VEGF antibody to the standard chemotherapy regimen has been shown to improve the overall survival (OS) of patients with advanced unresectable mCRC [8, 9]. However, so far comparative trials brought inconclusive outcomes [10, 11]. Panitumumab (PAN) is a fully human monoclonal antibody targeting the epidermal growth factor receptor (EGFR), approved in the USA and Europe for the treatment of RAS wild-type mCRC [12].
At ASCO 2022, Yoshino et al. presented results of the open-label, multicenter, phase 3 PARADIGM study (NCT02394795) which investigated the efficacy and safety of PAN plus mFOLFOX6 or bevacizumab (BEV) plus mFOLFOX6 in chemotherapy-naive patients with RAS-WT [13]. Eligible patients presented with an unresectable disease, had no previous chemotherapy, a good performance status (ECOG 0-1), at least one evaluable lesion, an adequate organ function and a life expectancy of at least 3 months. In total, 823 patients were randomized 1:1 to PAN plus mFOLFOX6 or BEV plus mFOLFOX6. In both study groups, around two-third of the patients had metastases in the liver. In the overall population, most patients (60%-67%) had a prior primary tumor resection.
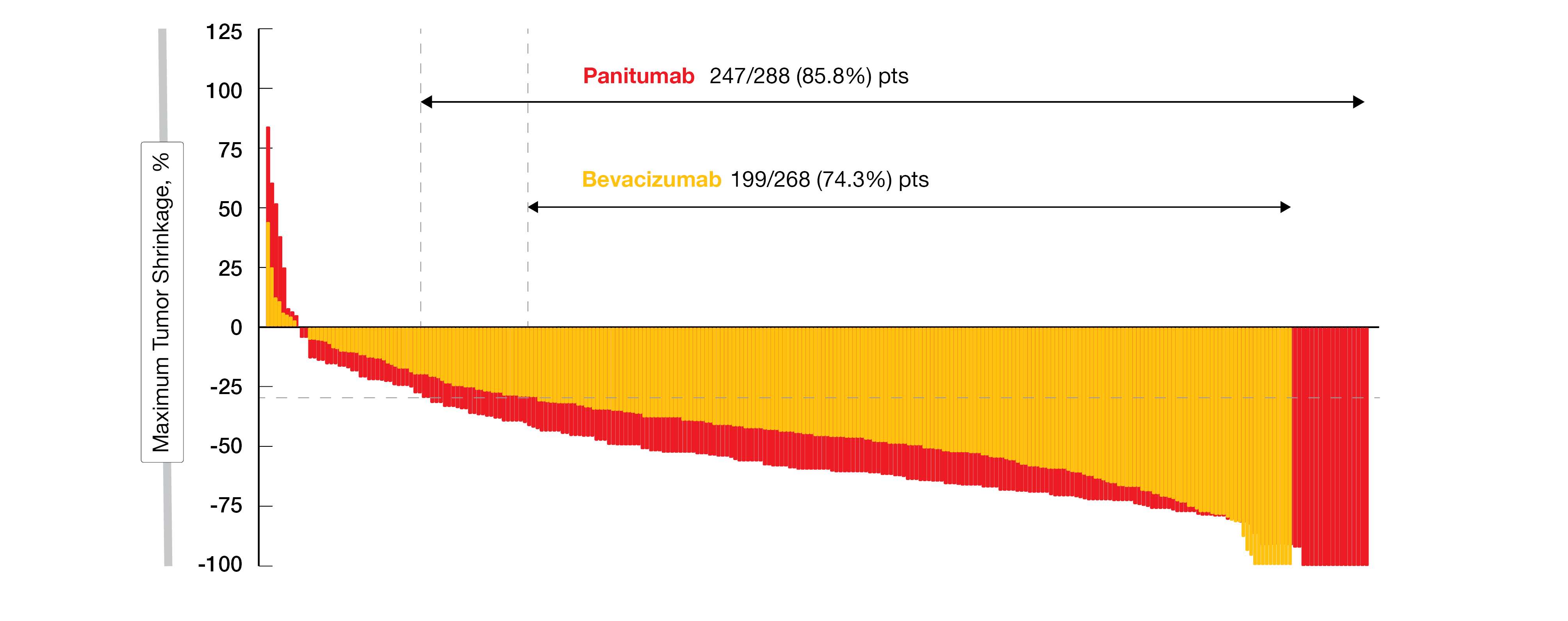
After a median follow-up of 61 months, the median OS in the left-sided population – the primary endpoint – was in favor of the investigational arm with PAN (37.9 vs 34.3 months; HR, 0.82; 95% CI, 0.68-0.99; p=0.031). Concerning the secondary endpoints, the PFS in the left-sided population attained 13.7 months with PAN plus mFOLFOX6 compared with 13.2 months in the comparator arm (stratified HR, 0.98; 95 % CI, 0.82-1.17). The response rate was 80.2% in the PAN arm versus 68.6% in the bevacizumab arm. Tumor shrinkage of more than 30%, indicating response per RECIST v1.1, was reported in 85.8% of patients in the PAN arm versus 74.3% in the BEV arm, with a median depth of response of 59% versus 44%, respectively (Figure 2). The disease control rate (DCR) was very similar in both arms (97.4% in the PAN arm vs 96.5% in the BEV arm). The median duration of response (mDoR) was 13.1 vs 11.2 months, while the curative resection (R0) rate attained 18.3% in the investigational arm versus 11.6% in the control arm. Clinical outcomes obtained for left-sided mCRC were comparable with the data presented for the overall population.
In total, grade ≥ 3 AEs occurred in 71.8% of patients in the PAN arm versus 64.9% in the BEV arm. SAEs related to the study treatment were experienced by 17.8% and 10.8% of patients in both study arms. In 23.8% of the PAN-treated patients and 18.4% of the BEV-treated patients, those AEs led to discontinuation of the treatment. The most common grade ≥3 AEs observed in the investigational/control arm were decreased neutrophil count (32%/35%), acne-like dermatitis (17%/0%) or peripheral sensory neuropathy (9%/10%). No new safety signals were reported.
The authors concluded that these clinical outcomes are supporting the use of panitumumab plus mFOLFOX6 as first-line therapy in patients with RAS-WT and left-sided mCRC.
Figure 2: Depth of response in the left-sided population of the PARADIGM trial (Horizontal dotted line at 30% indicates response per RECIST v1.1).
Novel coupled CAR T-cell therapy for mCRC
Although chimeric antigen receptor (CAR-T) therapy has proven efficacy in hematologic malignancies [14], there has been limited success in solid tumors [15]. The first clinical candidate from the CoupledCar® solid tumor platform has been introduced – GCC19CART. It has been designed to overcome the limitations of conventional CAR-T cells therapy by pairing solid tumor CAR-T cells with CD19 targeting CAR-T cells to amplify proliferation and activation of the solid tumor CAR-T component. Anti-CD19 CAR T-cell therapy demonstrated its efficacy in patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma [16]. Metastatic lesions of 70%-80% of subjects with CRCs are associated with guanylate cyclase-C (GCC). GCC19CART specifically targets GCC; recently, a phase 1 investigator-initiated dose escalation trial in patients with relapsed or refractory mCRC began in China [17].
In total, 21 subjects have been enrolled in this phase 1 study (ChiCTR2100053828) after positive screening for GCC expression by immunohistochemistry. All eligible subjects underwent leukapheresis, a single dose of lymphodepleting chemotherapy (fludarabine 30mg/m2, cyclophosphamide 300mg/m2) three days prior to infusion. Then a single infusion of GCC19CART at one of two preassigned doses (1×106 or 2×106 CAR T-cells/kg) was administered. Efficacy in this trial was determined with computed tomography (CT) or PET/CT according to RECIST v1.1 or PERCIST v1.0.
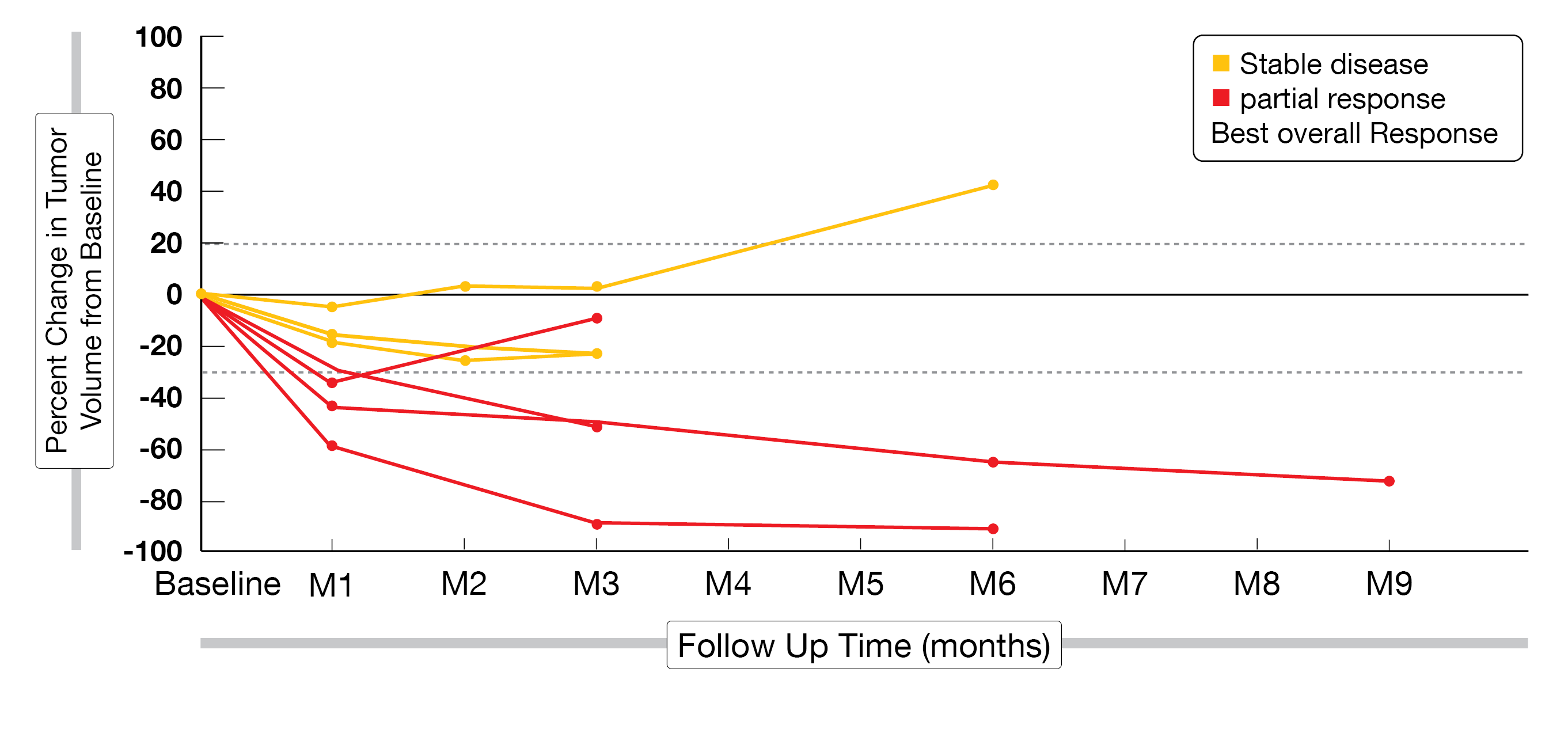
Overall, 13 subjects received the lower CAR T-cells dose and 8 subjects the higher dose. All 21 subjects had a one-month post-infusion imaging study available for review. The primary endpoint – combined ORR for both dose levels – was 28.6% (15.4% for dose level 1, 50% for dose level 2); two patients had a partial response (PR), while three more patients showed a partial metabolic response (PMR) in PET/CT scans with either stable disease or progressive disease for dose level 1 whereas 4 subjects demonstrated a PR and two a PMR with SD for dose level 2 (Figure 3). Combined safety data (dose 1 and dose 2) revealed that the most common grade ≥3 AEs were decreased lymphocyte count (85.7%), diarrhea (42.9%), decreased platelet count (28.6%) and decreased neutrophil count (23.8%).
This phase 1 study demonstrated that GCC19CART had a meaningful dose-dependent antitumoral activity and a tolerable safety profile in patients with relapsed or refractory metastatic CRC.
Figure 3: Change in tumor volume over time at a dose of 2×106 CAR T-cells/kg.
RGX-202-01 in second line advanced KRAS-mutated CRC
The activating KRAS mutation is found in 40%-45% of patients with CRC tumors [18]. RGX-202-01, a first-in-class oral inhibitor of the SLC6A8/CKB pathway, is currently being investigated in refractory CRC patients with KRAS-mutated CRC [19, 20]. RGX-202-01 does not just demonstrate single agent activity, it also exhibits synergistic activity with 5-FU in preclinical animal models, leading to a therapeutic combination of RGX-202-01 with 5-FU containing regimens such as FOLFIRI [21, 22]. Currently, FOLFIRI plus BEV is the most used second line treatment for mCRC in the United States, (ORR, 5%-15%; median PFS, 5-6 months; median OS, 12-18 months).
Currently, a phase 1b study (NCT03597581) is underway to evaluate the safety, PK/PD, and efficacy of RGX-202-01 in combination with the standard of care (SOC) FOLFIRI plus BEV in second-line CRC. In total, 19 patients with advanced or metastatic CRC who had a measurable disease according to RECIST v1.1 were analyzed (8 patients in the dose escalation and 11 patients in the dose expansion). Patients in the dose escalation cohorts received either 2,400 mg or 3,000 mg of RGX-202-01 orally (twice daily (BID) on days 1-28 of each 28-day cycle) combined FOLFIRI plus with BEV (IV 5 mg/kg) followed by irinotecan (180 mg/m² IV) concurrently with folinic acid (400 mg/m²), followed by 5-FU (2,400 mg/m² IV over 46 h, on days 1 and 15 of each 28-day cycle). Patients enrolled so far in the dose expansion phase received 3,000 mg of RGX-202-01 BID on days 1-28 of each 28-day cycle plus combined with FOLFIRI plus BEV.
Preliminary efficacy showed that patients with KRAS-mutated tumors experienced a durable clinical benefit with an ORR of 50% (Figure 4) and a median PFS of 11.8 months at the data cut-off date. The ORR and median PFS observed to date in patients with KRAS mutant tumors were superior to those with SOC FOLFIRI plus BEV alone. In this ongoing clinical trial, initial safety data demonstrated that there were no dose-limiting toxicities (DLTs) at the 2,400 mg or 3,000 mg BID dose. Grade 3 treatment-emergent AEs (TEAEs) were observed in 25% of patients, the most frequent being fatigue, hypertension, and rectal pain (25% each) at the 2,400 mg BID dose, as well as neutropenia, abdominal pain, or intestinal obstruction (13% each) at the 3,000 mg BID dose. Two patients experienced grade 4 TEAEs (sepsis and neutropenia) at the highest dose administered.
In this early phase clinical trial, the addition of RGX-202-01 to FOLFIRI plus BEV already provided a promising anti-tumoral activity and a favorable safety profile in KRAS-mutated advanced or metastatic CRC patients.
Figure 4: Best response according to RECIST v1.1 in all evaluable patients (n=17). One patient discontinued due to grade 2 allergic reaction, and another because of poor compliance.
Maintenance for patients with newly diagnosed CRC
There has been little to no advancement in the management of metastatic microsatellite stable colorectal cancer (MSS-CRC) in the past decade and available chemotherapy treatments are associated with cytotoxicity [23]. Recently, checkpoint inhibitors (CPIs) have shown benefit to a small subset of patients deficient in mismatch repair dMMR/microsatellite instabilityhi, whereas this advantage was not seen in MSS-CRC patients. The lack of neoantigen specific T-cells and immune infiltration associated with MSS-CRC may lead to the absence of clinical benefit from CPIs. To expand the number of patients with mCRC who may benefit from an immunotherapy even further, an individualized neoantigen vaccine that induces CD8 T-cells capable of tumor lysis is currently under development. Previously reported data from a Phase 1/2 study evaluating neoantigen vaccines in combination with CPIs in patients with previously treated mCRC demonstrated a molecular response (MR) rate (≥50% reduction in circulating tumor DNA [ctDNA] relative to baseline levels) in 4/9 (44%) patients: this outcome correlated with improvement in overall survival (OS) compared to those not showing a MR.
The randomized, open-label, multi-center phase 2/3 GO-010 study (NCT05141721) aims to evaluate the efficacy and safety of 2 neoantigen-containing vectors (GRT-C901-adenoviral vector plus GRT-R902-self-amplifying mRNA vector) as prime/boost combined with CPIs (add-on to fluoropyrimidine/bevacizumab [BEV] following first-line therapy with fluoropyrimidine/oxaliplatin [FOLFOX]/BEV) in patients with mCRC [24]. The primary objective of the phase 2 study is to assess the antitumor activity based on MR, whereas the phase 3 study aims to assess antitumor activity based on PFS per iRECIST. Patients will be randomized 1:1 with up to 90 patients being recruited in phase 2 and up to 226 in the phase 3 trial. This study will be conducted in two stages, vaccine production and study treatment, respectively. In the vaccine production phase, where patients receive up to 24 weeks FOLFOX/BEV (induction phase), neoantigen prediction will be performed using a tumor biopsy and Gritstone’s EDGETM neoantigen prediction model. In the study treatment phase, patients in the control arm will continue with maintenance therapy (fluoropyrimidine/BEV), whereas patients in the vaccine arm will add the vaccine regimen (a total of 6 intramuscular injections of GRT-C901/GRT-R902 plus 30 mg ipilimumab subcutaneously [SC] co-administered only with the first dose of the cancer vaccines), as well as atezolizumab (1680 mg, intravenously [IV], once every 4 weeks [q4w] for up to 2 years) to the maintenance therapy. The vaccine will be evaluated using imaging, ctDNA, safety, immunogenicity, and exploratory biomarker analysis. Clinical benefit will be assessed in the 1L maintenance setting, using a real-time non-invasive ctDNA monitoring system as novel biomarker for tumor response. The key outcome will be to evaluate the correlation of ctDNA reduction with the improvement in clinical outcomes such as the immune-based PFS (iPFS).
Dose escalation trial in patients with advanced solid tumors expressing B7-H6
Several solid tumor types express B7-H6, a member of the B7 family of immune receptors, whereas little to no expression of B7-H6 is seen in normal tissue [25, 26]. A novel immunoglobin G-like bi-specific T-cell engager – BI-765049 – has been developed to simultaneously bind to B7-H6 tumor cells and CD-3 on T-cells; this binding results in cytotoxic synapse formation local activation and proliferation of T-cells, as well as cytokine secretion, converting a non-inflamed (cold) tumor environment into an inflamed (hot) tumor environment. Previously published preclinical studies have demonstrated that monotherapy with BI-765049 induced a dose-dependent anti-tumor activity in humanized in vivo CRC models, as well as an infiltration of T-cells [27]. BI-765049 is currently under clinical investigation in a Phase 1 trial as monotherapy or in combination with the PD-1 inhibitor ezabenlimab in patients with CRC or other B7-H6 positive tumors in several indications (non-small cell lung cancer [NSCLC], as well as head and neck squamous cell- [HNSCC], hepatocellular-, pancreatic-, gastric- or colorectal [CRC] carcinoma) [28].
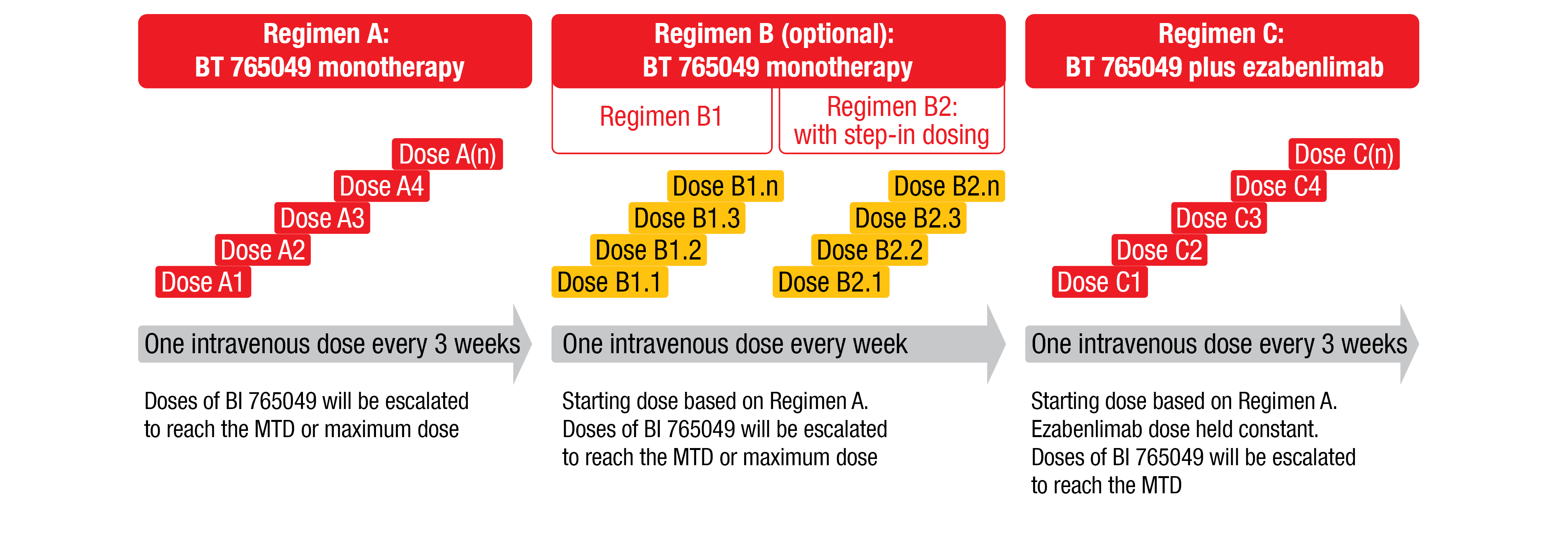
The first open-label dose-escalation trial (NCT04752215) of BI-765049 +/- ezabenlimab already started in January 2022 to recruit adult patients with confirmed, advanced, unresectable and/or metastatic CRC, or patients with confirmed B7-H6-positive NSCLC, HNSCC, hepatocellular-, gastric-, or pancreatic carcinoma. Moreover, eligible patients should have at least 1 evaluable lesion (according to RECIST v1.1 outside of the central nervous system), adequate liver, bone marrow and renal functions, as well as a good performance status (ECOG 0/1). The co-primary endpoints of the study are the determination of the maximum tolerated dose (MTD) and the recommended dose for expansion based on the number of patients with DLTs during the MTD evaluation period. The secondary endpoints include pharmacokinetic parameters after first and multiple doses in all regimens, as well as ORR based on RECIST v1.1. The study is designed to assess up to four intravenous drug regimens (Figure 5). Eligible patients receive the treatment for a maximum duration of 36 months or until confirmation of progressive disease, unacceptable toxicity, or other withdrawal reason. The enrollment goal is approximately 120 patients.
Figure 5: Study design of the first-in-human phase I dose-escalation trial of B7-H6/CD3 T-cell engager BI-765049 ± ezabenlimab in patients with advanced solid tumors expressing B7-H6.
REFERENCES
- Siegel, RL, et al., Cancer statistics, 2020. CA Cancer J Clin 2020; 70(1): 7-30.
- NCCN guidelines for colon cancer. 2021 June 13, 2022]; version 2.2021:[
- Yoshino, T, et al., Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018; 29(1): 44-70.
- Cremolini, C, et al., Activity and Safety of Cetuximab Plus Modified FOLFOXIRI Followed by Maintenance With Cetuximab or Bevacizumab for RAS and BRAF Wild-type Metastatic Colorectal Cancer: A Randomized Phase 2 Clinical Trial. JAMA Oncol 2018; 4(4): 529-536.
- Ychou, M, et al., Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br J Cancer 2022; 126(9): 1264-1270.
- Modest, DP, et al., FOLFOXIRI Plus Panitumumab As First-Line Treatment of RAS Wild-Type Metastatic Colorectal Cancer: The Randomized, Open-Label, Phase II VOLFI Study (AIO KRK0109). J Clin Oncol 2019; 37(35): 3401-3411.
- Cremolini, C, et al., Modified FOLFOXIRI plus panitumumab (mFOLFOXIRI/PAN) versus mFOLFOX6/PAN as initial treatment of patients with unresectable RAS and BRAF wild-type metastatic colorectal cancer (mCRC): Results of the phase III randomized TRIPLETE study by GONO. J Clin Oncol 2022; 40(suppl 16; abstr LBA3505).
- Heinemann, V, et al., FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15(10): 1065-75.
- Venook, AP, et al., Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. Jama 2017; 317(23): 2392-2401.
- Arnold, D, et al., Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017; 28(8): 1713-1729.
- Stintzing, S, et al., FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016; 17(10): 1426-1434.
- Vectibix SmPC. 2022; Available from: https://www.ema.europa.eu/en/documents/product-information/vectibix-epar-product-information_en.pdf.
- Yoshino, T, et al., Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. J Clin Oncol 2022; 40(suppl 17; abstr LBA1).
- Sterner, RC, et al., CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 2021; 11(4): 69.
- Bell, M, et al., CAR T cell therapy for solid tumors: Fatal attraction requires adhesion. Med (N Y) 2022; 3(6): 353-354.
- Abramson, JS, Anti-CD19 CAR T-Cell Therapy for B-Cell Non-Hodgkin Lymphoma. Transfus Med Rev 2020; 34(1): 29-33.
- Cui, J, A phase 1 dose-escalation study of GCC19 CART a novel coupled CAR therapy for subjects with metastatic colorectal cancer. J Clin Oncol 2022; 40(suppl 16; Abstr 3582).
- Jones, RP, et al., Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer 2017; 116(7): 923-929.
- Hendifar, AE, Phase 1b study of RGX-202-01, a first-in-class oral inhibitor of the SLC6A8/CKB pathway, in combination with FOLFIRI and bevacizumab (BEV) in second-line advanced colorectal cancer (CRC). J Clin Oncol 2022; 40(suppl 16; Abstr 3579).
- Loo, JM, et al., Extracellular metabolic energetics can promote cancer progression. Cell 2015; 160(3): 393-406.
- Kurth, I, et al., Therapeutic targeting of SLC6A8 creatine transporter suppresses colon cancer progression and modulates human creatine levels. Sci Adv 2021; 7(41): eabi7511.
- Bendell, JC, Phase I monotherapy dose escalation of RGX-202, a first-in-class oral inhibitor of the SLC6a8/CKB pathway, in patients with advanced gastrointestinal (GI) solid tumors. J Clin Oncol 2020; 38(suppl 15; Abstr 3504).
- Biller, LH, et al., Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. Jama 2021; 325(7): 669-685.
- Hecht, JR, Phase 2/3, randomized, open-label study of an individualized neoantigen vaccine (selfamplifying mRNA and adenoviral vectors) plus immune checkpoint blockade as maintenance for patients with newly diagnosed metastatic colorectal cancer (GRANITE). J Clin Oncol 2022; 40(suppl 16; abstr TPS3635).
- Boehringer Ingelheim, data on file. 2022.
- Brandt, CS, et al., The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med 2009; 206(7): 1495-503.
- Hipp, S. ACCR Annual Meeting 2021; Abstr 53.
- Falchook, G, et al., A first-in-human phase I dose-escalation trial of the B7-H6/CD3 T-cell engager BI 765049 ± ezabenlimab (BI 754091) in patients with advanced solid tumors expressing B7-H6. J Clin Oncol 2022; 40(suppl 16; abstr TPS3175).
© 2022 Springer-Verlag GmbH, Impressum
More posts
New therapeutic options being currently investigated in advanced or metastatic colorectal cancer
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, and it is the fourth most frequent cancer diagnosis.A current treatment option for RAS and BRAF wild-type (WT) metastatic colorectal cancer (mCRC) is the chemotherapy doublet (FOLFOX/FOLFIRI) with an anti-EGFR monoclonal antibody (cetuximab or panitumumab).
An update and future directions in advanced gastric or gastrointestinal junction cancer (G/GEJC)
With more than 1 million newly diagnosed cases in 2020, gastric cancer (GC) is the fifth most frequent cancer; it was also the third leading cause of cancer-related death worldwide. Gastroesophageal junction (GEJ) cancer concerns a form of gastric cancer developing around the digestive tract where esophagus and stomach connect; in the last years, the prevalence of GEJ constantly increased.
Innovative combinations in esophageal squamous cell carcinoma
Each year, esophageal cancer (EC) is responsible for more than half a million deaths worldwide. Among them, esophageal squamous cell carcinoma (ESCC) accounts for the vast majority (~ 85 %) of EC incidences . At diagnosis, 70 % of ESCC is unresectable [3] and the 5-year survival rate is limited (30 % - 40 %). Patients with advanced or metastatic ESCC have a poor prognosis; their overall survival (OS) after standard first-line chemotherapy is limited to less than a year and other treatment options are scarce.
Novel agents or combinations in recurrent or metastatic nasopharyngeal cancer
Nasopharyngeal cancer (NPC) is a rare malignancy with an incidence of approximately 133,000 annually worldwide, resulting in about 80,000 deaths per year. Whereas early-stage and locally advanced NPC have a good prognosis, treatment of recurrent or metastatic nasopharyngeal cancer is a challenging; it is thus associated with a poor prognosis, especially in patients who have failed two or more lines of systemic therapy, with a median progression-free survival (mPFS) of seven months and median overall survival (mOS) of 22 months.
Preface ASCO Solid Tumor 2022
After 2 years of the COVID-19 pandemic, the Annual Meeting of the American Society of Clinical Oncology (ASCO), was held in Chicago, USA, and virtually from 3rd–7th June 2022.As always, the very much-anticipated event brought leading experts from across the globe together to learn and discuss the groundbreaking updates and scientific advancements which were covered in more than 2,000 abstracts, along with 85 livestream sessions, and more than 2,500 poster presentations.