Changing paradigms in the management of mantle cell lymphoma
Mantle cell lymphoma (MCL) is a rare, heterogenous and generally aggressive subtype of B-cell non-Hodgkin lymphoma that remains incurable in the majority of cases. Median survival in non-trial patients has been estimated at 3 to 5 years [1]. First-line therapy usually consists of chemoimmunotherapy, while both immunochemotherapy and targeted agents are recommended in relapsed disease [2]. However, trials increasingly challenge the role of chemotherapy against the novel agents, especially in the front-line setting.
OAsis: venetoclax, ibrutinib and obinutuzumab
Venetoclax combined with ibrutinib and obinutuzumab was assessed in the phase I/II, non-randomized OAsis trial that included patients with newly diagnosed and relapsed/refractory MCL. Le Gouill et al. reported the results for 15 untreated patients enrolled in Cohort C [3]. The treatment schedule was ibrutinib 560 mg once daily until progression, obinutuzumab 1 g administered on days 1, 8 and 15 of cycle 1 and on day 1 thereafter (from cycle 8, it was given every 2 cycles), and venetoclax 400 mg daily after dose ramp-up in cycle 2. Treatment duration for both obinutuzumab and venetoclax was limited to 2 years. A considerable proportion of patients in this small cohort had high-risk cytogenetics such as TP53 mutation or 17p deletion.
The triple combination was generally well tolerated, with most AEs from cycle 1 to 6 being grades 1 and 2. Complete remissions emerged early; after 2 cycles, 53 % of patients achieved CR or unconfirmed CR according to the Cheson 99 criteria. At cycle 6, this was 80 %, and the ORR was 93 %. All patients evaluable for MRD (n = 12) obtained MRD negativity in the peripheral blood at cycle 3 and remained MRD-negative in both blood and bone marrow at the end of cycle 6. After a follow-up of 14 months, 14 patients remained in CR and on treatment. The 1-year PFS rate was 93.3 %, and all patients were alive at 2 years.
Although only a small cohort was assessed, the high complete response rate reported compares favorably even to standard immunochemotherapy induction and provides further evidence of the high potency of venetoclax/ibrutinib-based combinations in MCL. The MRD negativity rates, which compare favorably to those observed in the relapsed setting, suggest that the treatment may be most beneficial when given upfront. According to the authors’ conclusion, the ibrutinib/obinutuzumab/venetoclax triple therapy is a highly attractive option for untreated MCL patients regardless of age and deserves to be investigated in a larger trial. The OAsis II study assessing venetoclax, ibrutinib plus an anti-CD20 antibody compared to ibrutinib plus an anti-CD20 antibody in the frontline setting will start in late 2020.
Genetic aberrations as markers of chemoresistance
MCL typically involves a large number of recurrent molecular aberrations. Given the lack of reliable markers of chemoresistance at the time of diagnosis, Malarikova et al. evaluated the prognostic impact of 7 recurrent gene aberrations (TP53, CDKN2A, ATM, BCL2, MYC, RB1 and CDK4) in a real-world cohort of 126 newly diagnosed consecutive MCL patients with bone marrow involvement ≥ 5 % [4].
The investigators found that the total number of gene aberrations correlated with shorter survival and is therefore a strong predictor of outcome. Here, the largest difference was seen between any two aberrations and any isolated aberration. CDKN2A deletion was observed exclusively in the context of other aberrations, which suggests that it represents a later event. Concurrent deletion and/or mutation of TP53 and deletion of CDKN2A represented the most significant predictor of short event-free survival (Figure) and OS. The investigators noted that concurrent aberration of TP53 and CDKN2A is a new, simple and relevant index of chemoresistance in MCL. These patients should be offered innovative anti-lymphoma therapy and upfront consolidation with allogeneic stem cell transplantation.
Figure: Shortest event-free survival in patients showing both TP53 aberrations and deletion of CDKN2A
Real-world evidence for ibrutinib
In the relapsed setting, the role of novel agents, especially BTK inhibitors, is increasingly being established [1]. Real-world data from a national audit database in the United Kingdom for patients receiving second-line ibrutinib show that this agent is both effective and well tolerated in frail patients unsuitable for most standard frontline immunochemotherapy regimens [5]. All patients receiving second-line ibrutinib were retrospectively divided into three cohorts using first-line therapy as a surrogate marker for overall fitness.
In the group treated with less intensive regimens (i.e., rituximab plus chlorambucil) prior to ibrutinib, second-line ibrutinib gave rise to a median PFS of 9.8 months compared to the median PFS with frontline therapy of only 4.0 months. The median OS from the start of ibrutinib was 10.7 months. Seventy-one percent of these patients responded, and almost 10 % obtained CR. Ibrutinib was generally well tolerated in the cohort of frail patients, and disease progression constituted the most common reason for treatment discontinuation. The more durable responses observed with second-line ibrutinib suggest that this patient group may benefit from frontline BTK inhibitor therapy, and further exploration in clinical trials is warranted.
Zanubrutinib in the second and later lines
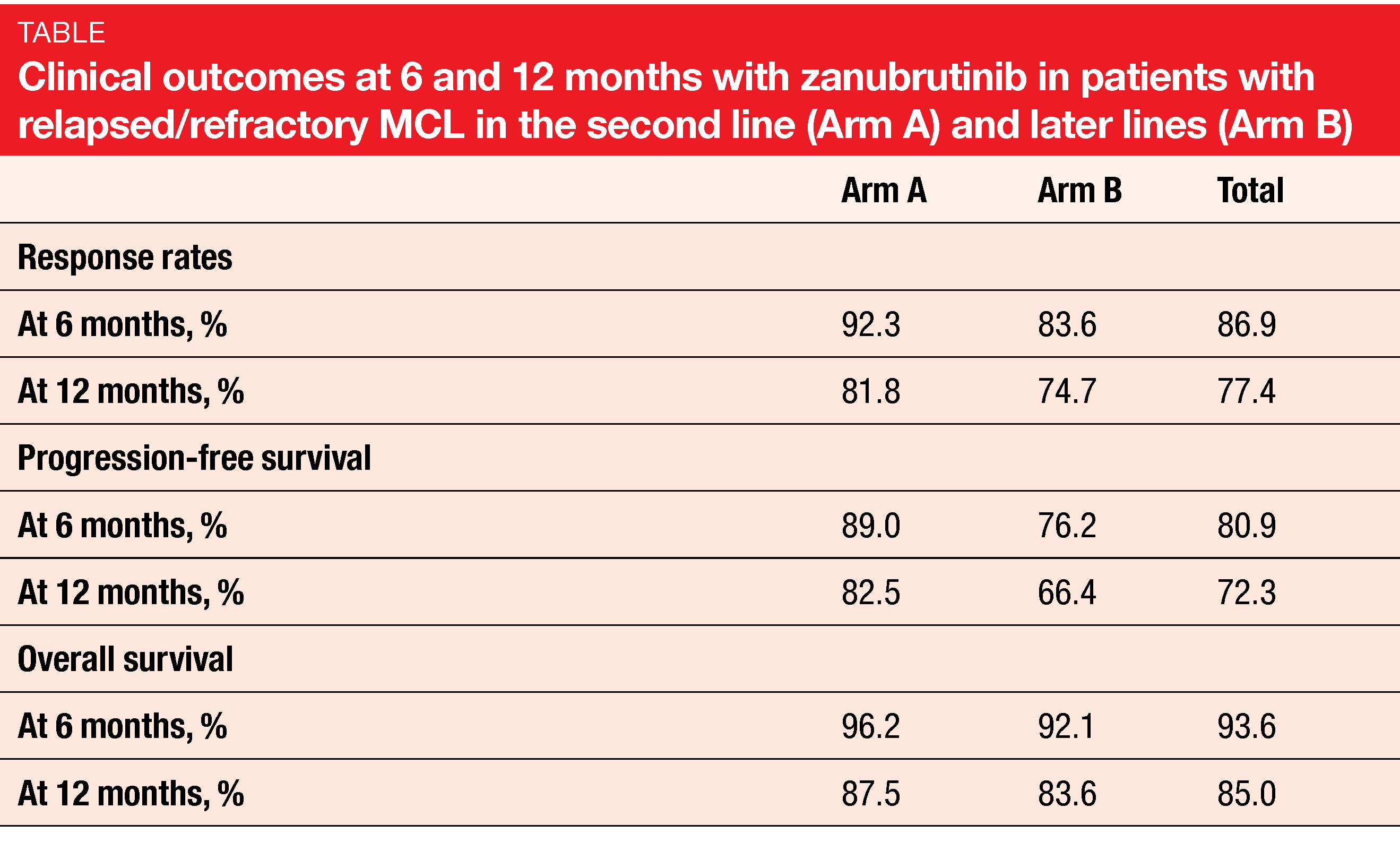
The specific, potent next-generation BTK inhibitor zanubrutinib has shown complete and sustained 24-hour BTK occupancy in both blood and lymph node biopsies and elicits durable responses in patients with non-Hodgkin lymphoma including MCL [6, 7]. A phase II study showed an ORR of 84 % in zanubrutinib-treated patients with relapsed/refractory MCL, with 78 % obtaining complete remissions [7]. At EHA 2020, Zhou et al. presented pooled clinical outcomes in patients with relapsed/refractory MCL who received zanubrutinib in phase I (NCT02343120) and phase II (NCT03206970) trials [8]. Forty-one and 71 patients were treated in the second-line (Arm A) and later-line (Arm B) settings, respectively. Imbalances in baseline characteristics between groups with different prior lines of therapy were adjusted using inverse propensity score weighting.
Complete responses occurred significantly more often in Arm A than in Arm B (74.6 % vs. 61.1 %). The adjusted odds of achieving CR when treated with zanubrutinib in the second line were 3.4 times as high as in later lines. Likewise, Arm A fared better with respect to duration of response as well as PFS and OS rates at 6 and 12 months (Table). In general, patients in Arm A also showed an improved safety profile of zanubrutinib, particularly regarding AEs of special interest such as diarrhea, major hemorrhage, and atrial fibrillation/flutter. The rates of discontinuation due to AEs were low in both arms.
REFERENCES
- Rule S, The modern approach to mantle cell lymphoma. Hematol Oncol 2019; 37 Suppl 1: 66-69
- Dreyling M et al., Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28 (Supplement 4): iv62-iv71
- Le Gouill S et al., Ibrutinib, venetoclax plus obinutuzumab in newly diagnosed mantle cell lymphoma patients: OAsis phase I/II trial. EHA 2020, abstract S228
- Malarikova D et al., Concurrent TP53 and CDKN2A gene aberrations in newly diagnosed MCL correlate with chemoresistance and call for innovative upfront therapy. EHA 2020, abstract EP1166
- Johns S et al., Ibrutinib as second-line therapy is well tolerated and efficacious in frail patients with relapsed/refractory mantle cell lymphoma who are unsuitable for standard front-line therapies. EHA 2020, abstract EP1177
- Tam CS et al., Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019; 134(11): 851-859
- Song Y et al., Zanubrutinib in patients with relapsed/refractory mantle cell lymphoma. International Conference on Malignant Lymphoma 2019, abstract 15
- Zhou K et al., Outcomes of relapsed/refractory MCL patients treated with zanubrutinib monotherapy in the second line and in later lines: a pooled analysis from 2 studies. EHA 2020, abstract EP1169
More posts
Patient and disease characteristics in a small CAD cohort
A retrospective analysis hints at the wide range of cold agglutinin disease (CAD) clinical behavior. Koudouna et al. investigated the characteristics of 8 patients with CAD at the time of diagnosis [1]. Median age was 62 years, and 5 patients were women. Hematologic malignancies constituted 50 % of underlying medical conditions; in 37 %, hepatitis B/C was the associated disease, and in 13 %, autoimmune disorders.
Cold agglutinin disease: on the road to new insights and potential treatment options
Cold agglutinin disease (CAD) is a rare type of autoimmune hemolytic anemia (AIHA) elicited by cold-sensitive antibodies including cold agglutinins. Ninety percent of cold agglutinins belong to the IgM kappa category and bind to red blood cell surface antigens at temperatures of ≤ 37 °C, thus inducing hemolysis.
Paroxysmal nocturnal hemoglobinuria: improving outcomes with novel strategies
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, potentially life-threatening clonal hematopoietic stem cell disorder characterized by hemolytic anemia, bone marrow failure, thrombosis, and peripheral blood cytopenia. The disease results from an acquired loss-of-function mutation of the PIGA gene involved in the synthesis of the glycosylphosphatidylinositol-anchored complement inhibitors CD55 and CD59.
Targeted approaches in various B-cell malignancies
BTK inhibitors are active in many B-cell malignancies such as mantle cell lymphoma, CLL and Waldenström’s macroglobulinemia, but also in diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and marginal zone lymphoma (MZL). Zanubrutinib is currently being assessed in pivotal phase II and III studies in all of these indications.
Changing paradigms in the management of mantle cell lymphoma
Mantle cell lymphoma (MCL) is a rare, heterogenous and generally aggressive subtype of B-cell non-Hodgkin lymphoma that remains incurable in the majority of cases. Median survival in non-trial patients has been estimated at 3 to 5 years. First-line therapy usually consists of chemoimmunotherapy, while both immunochemotherapy and targeted agents are recommended in relapsed disease.
Optimizing timing, efficacy and tolerability in chronic lymphocytic leukemia
In both treatment-naïve and relapsed/refractory patients with chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia (SLL), inhibition of Bruton’s tyrosine kinase (BTK) represents a treatment standard as it has improved clinical outcomes. Compared to the first-generation agent ibrutinib, the second-generation, highly selective BTK inhibitor acalabrutinib shows minimal off-target kinase inhibition, thus potentially offering an optimized safety profile.

![Mantle cell lymphoma (MCL) is a rare, heterogenous and generally aggressive subtype of B-cell non-Hodgkin lymphoma that remains incurable in the majority of cases. Median survival in non-trial patients has been estimated at 3 to 5 years [1]. First-line therapy usually consists of chemoimmunotherapy, while both immunochemotherapy and targeted agents are recommended in relapsed disease [2]. However, trials increasingly challenge the role of chemotherapy against the novel agents, especially in the front-line setting. OAsis: venetoclax, ibrutinib and obinutuzumab Venetoclax combined with ibrutinib and obinutuzumab was assessed in the phase I/II, non-randomized OAsis trial that included patients with newly diagnosed and relapsed/refractory MCL. Le Gouill et al. reported the results for 15 untreated patients enrolled in Cohort C [3]. The treatment schedule was ibrutinib 560 mg once daily until progression, obinutuzumab 1 g administered on days 1, 8 and 15 of cycle 1 and on day 1 thereafter (from cycle 8, it was given every 2 cycles), and venetoclax 400 mg daily after dose ramp-up in cycle 2. Treatment duration for both obinutuzumab and venetoclax was limited to 2 years. A considerable proportion of patients in this small cohort had high-risk cytogenetics such as TP53 mutation or 17p deletion. The triple combination was generally well tolerated, with most AEs from cycle 1 to 6 being grades 1 and 2. Complete remissions emerged early; after 2 cycles, 53 % of patients achieved CR or unconfirmed CR according to the Cheson 99 criteria. At cycle 6, this was 80 %, and the ORR was 93 %. All patients evaluable for MRD (n = 12) obtained MRD negativity in the peripheral blood at cycle 3 and remained MRD-negative in both blood and bone marrow at the end of cycle 6. After a follow-up of 14 months, 14 patients remained in CR and on treatment. The 1-year PFS rate was 93.3 %, and all patients were alive at 2 years. Although only a small cohort was assessed, the high complete response rate reported compares favorably even to standard immunochemotherapy induction and provides further evidence of the high potency of venetoclax/ibrutinib-based combinations in MCL. The MRD negativity rates, which compare favorably to those observed in the relapsed setting, suggest that the treatment may be most beneficial when given upfront. According to the authors’ conclusion, the ibrutinib/obinutuzumab/venetoclax triple therapy is a highly attractive option for untreated MCL patients regardless of age and deserves to be investigated in a larger trial. The OAsis II study assessing venetoclax, ibrutinib plus an anti-CD20 antibody compared to ibrutinib plus an anti-CD20 antibody in the frontline setting will start in late 2020. Genetic aberrations as markers of chemoresistance MCL typically involves a large number of recurrent molecular aberrations. Given the lack of reliable markers of chemoresistance at the time of diagnosis, Malarikova et al. evaluated the prognostic impact of 7 recurrent gene aberrations (TP53, CDKN2A, ATM, BCL2, MYC, RB1 and CDK4) in a real-world cohort of 126 newly diagnosed consecutive MCL patients with bone marrow involvement ≥ 5 % [4]. The investigators found that the total number of gene aberrations correlated with shorter survival and is therefore a strong predictor of outcome. Here, the largest difference was seen between any two aberrations and any isolated aberration. CDKN2A deletion was observed exclusively in the context of other aberrations, which suggests that it represents a later event. Concurrent deletion and/or mutation of TP53 and deletion of CDKN2A represented the most significant predictor of short event-free survival (Figure) and OS. The investigators noted that concurrent aberration of TP53 and CDKN2A is a new, simple and relevant index of chemoresistance in MCL. These patients should be offered innovative anti-lymphoma therapy and upfront consolidation with allogeneic stem cell transplantation.](https://memoinoncology.com/wp-content/uploads/2020/08/Graph-8-eha-2020-en.png)