Encouraging findings in NTRK-, ROS1– and ALK-positive lung cancer
TRK inhibition: larotrectinib
Neutrotrophic receptor tyrosine kinase (NTRK) gene fusions occur in a wide array of different cancers including rare entities such as infantile fibrosarcoma, but also in common tumors including melanoma, colon cancer, and lung cancer [1]. Their incidence in lung cancer is estimated at 0.2 % to 3.3 % [1, 2]. The highly selective, oral, CNS-active TRK inhibitor larotrectinib has already been approved by the US Food and Drug Administration for the treatment of adult and pediatric patients with solid tumors showing NTRK fusions. Drilon et al. presented pooled data from 11 patients with TRK-fusion–positive lung cancer who had received larotrectinib in a phase I study and a phase II basket trial conducted in advanced solid tumors [3]. Five patients had previously undergone 1 or 2 systemic therapies, and in 5 cases, 3 or more therapies had been administered. The treatment consisted of larotrectinib 100 mg BID continuously. Indeed, the analysis demonstrated activity of larotrectinib in advanced lung cancers harboring TRK fusions. Seven patients (71 %) responded, with one and four experiencing complete remissions (CR) and partial remissions (PR), respectively. In two cases, disease stabilization occurred. None of the patients developed primary progressive disease. The median duration of response had not been reached at the time of the analysis. Furthermore, larotrectinib showed intracranial activity. A female patient who experienced confirmed PR developed near complete intracranial remission of multiple cerebral lesions. Larotrectinib was well tolerated in the entire data set including patients without lung cancer. Fatigue, dizziness and nausea occurred most frequently. The majority of AEs were low-grade events. Dose reductions became necessary in no more than 9 % of the total study population, and the discontinuation rate was low at < 1 %. Overall, these results support the routine molecular testing for TRK fusions in patients with NSCLC.
Meaningful responses with entrectinib
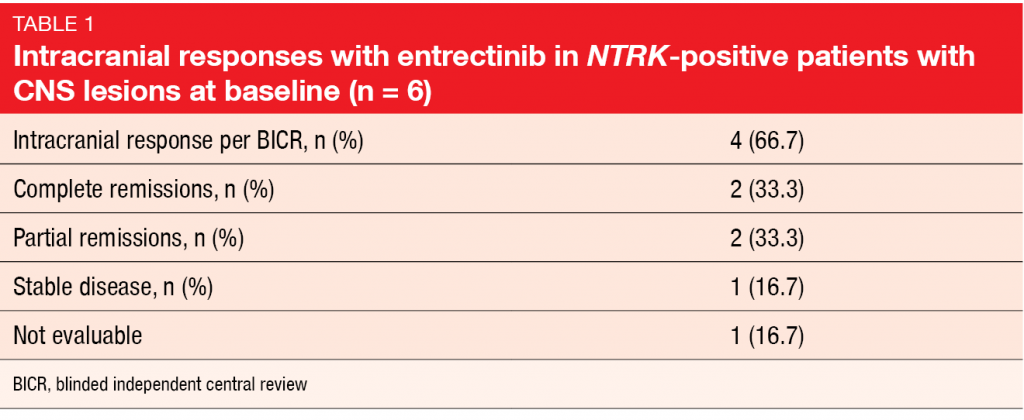
Another oral, selective, CNS-active TRK inhibitor is entrectinib that also targets ROS1 and ALK. Paz-Ares et al. reported an integrated analysis of adult patients with NTRK-fusion–positive, TRK-inhibitor–naïve solid tumors included in the ALKA-372-001, STARTRK-1 and STARTRK-2 trials [4]. ALKA-372-001 and STARTRK-1 were phase I dose-escalation studies, while the global phase II STARTRK-2 trial assessed entrectinib 600 mg/day. Out of 54 patients with various NTRK-fusion–positive solid tumors enrolled in these trials, 10 had lung cancer. Among these, six patients showed brain metastases at the time of study inclusion. Three had received one prior systemic treatment line and another 3 had been treated with ≥ 2 lines. Entrectinib induced clinically meaningful, durable systemic and intracranial responses. According to blinded independent central review, ORR was 57.4 % in the total population with different NTRK-fusion–positive solid tumors. For those with NSCLC, ORR was 70.0 %, and median duration of response had not been reached yet. One patient achieved CR, while 6 obtained PR, and disease stabilized in one case. Median PFS was 14.9 months. The analysis of the patients with brain metastases at baseline showed an intracranial response rate of 66.7 %. Two obtained CR (Table 1). Entrectinib was well tolerated; AEs were mainly graded as 1 or 2. Dysgeusia occurred as the most common toxicity with an incidence rate of 47.1 %, followed by constipation, fatigue, and diarrhea. Most AEs were managed with dose interruptions or reductions, and at 4.4 %, the discontinuation rate was low.
Entrectinib activity in ROS1-positive disease
In ROS1-positive lung cancer, the ALK/ROS1/MET inhibitor crizotinib has been established as the standard of care. However, an unmet need results from the fact that CNS is a common first site of progression in crizotinib-treated patients with ROS1-positive NSCLC [5]. Therefore, the introduction of a CNS-penetrant ROS1 inhibitor in the first-line setting appears desirable. Compared to crizotinib, entrectinib has shown higher potency regarding ROS1 inhibition in preclinical studies [6]. Entrectinib demonstrated clinical activity in multiple tumor histologies including primary brain tumors and secondary CNS metastases [7]. The ALKA-372-001, STARTRK-1 and STARTRK-2 trials evaluating entrectinib included a total of 53 ROS1-inhibitor–naïve patients with ROS1-positive NSCLC. Out of these, 23 had CNS lesions at baseline. An integrated analysis of these 53 patients revealed clinically meaningful and durable systemic and intracranial responses with entrectinib [8]. The systemic response was independent of the presence of baseline brain metastases. Objective response rates were 73.9 % and 80 % in patients with and without CNS lesions, respectively. Median duration of response was 12.6 and 24.6 months, respectively, and median PFS was 13.6 and 26.3 months, respectively. Three patients (10.0 %) of those without CNS disease achieved CR. For intracranial activity according to independent central review, it was shown that 55 % of 20 patients with baseline brain metastases responded, with 20 % and 35 % obtaining CR and PR, respectively. Intracranial responses lasted for a median of 12.9 months. Median OS had not been reached yet. The pooled safety population included 134 patients who received entrectinib without necessarily being TKI-naïve. Entrectinib proved tolerable in the ROS1-positive setting, with predominantly low-grade AEs that showed amenability to successful management by means of dose interruptions/reductions. The most common treatment-related AEs included dysgeusia, dizziness and constipation. In 4.5 % only, treatment had to be discontinued due to AEs.
PROFILE 1001: a new benchmark for OS
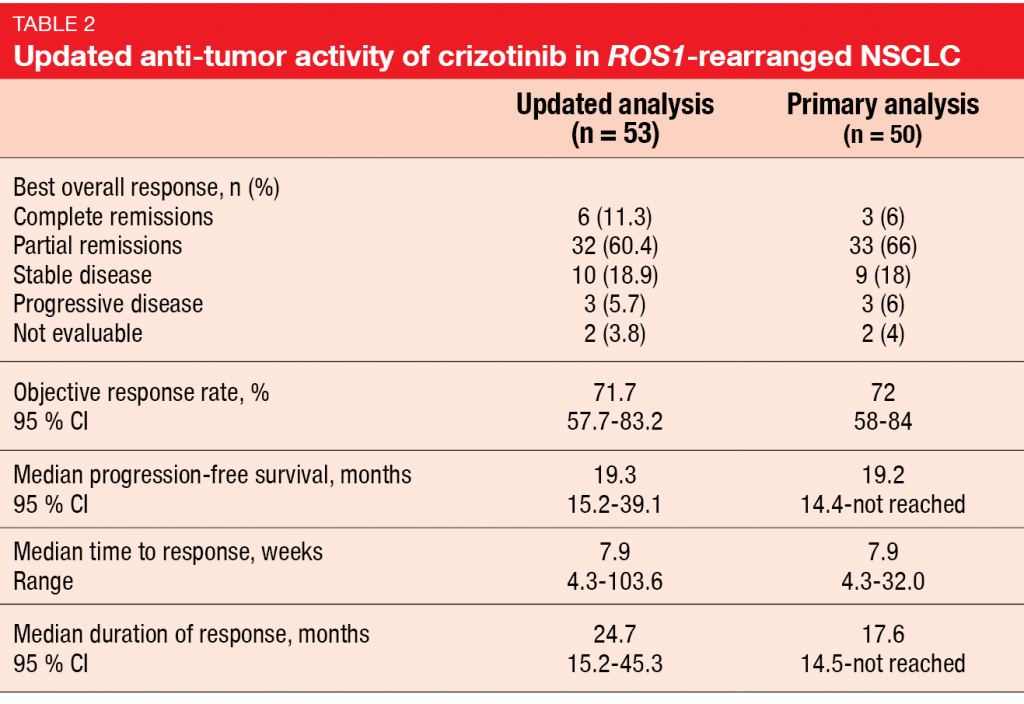
Updated results for crizotinib in ROS1-rearranged disease from the ROS1 expansion cohort of the PROFILE 1001 study were presented at ELCC 2019 by Shaw et al. [9]. The primary analysis of the PROFILE 1001 trial that was published in 2014 after a median follow-up of 16 months had shown marked anti-tumor activity [10]. Crizotinib 250 mg BID led to an ORR of 72 %, median duration of response of 17.6 months, and median PFS of 19.2 months. Median OS had not been reached yet. Based on these data, crizotinib was approved for ROS1-rearranged advanced NSCLC in many countries. After a median follow-up of 62.6 months for OS, median OS in the expansion cohort of PROFILE 1001 was 51.4 months in a total of 53 patients most of whom had received at least one prior treatment in the advanced setting. One-year and 4-year OS rates amounted to 79 % and 51 %, respectively. Survival did not differ according to ROS1 fusion partners, although the number of patients with each type of ROS1 rearrangement was small, and further studies are needed. Updated ORR was consistent with the results obtained at the time of the primary analysis, as was updated PFS (Table 2). However, updated duration of response, at 24.7 months, exceeded the result previously reported. Moreover, a greater proportion of patients had achieved CR compared to the primary analysis, probably due to longer exposure. Long-term crizotinib treatment did not give rise to any new safety signals. No patient developed treatment-related AEs requiring permanent treatment discontinuation. The authors noted that this analysis provides a new benchmark for OS in ROS1-rearranged advanced NSCLC. Considering the outstanding survival rate, this might be one of the few types of lung cancer that could be considered a chronic disease. Also, the data support the continued use of crizotinib in the treatment of patients with this molecular subtype of lung cancer.
First interim analysis of the ALTA-1L study
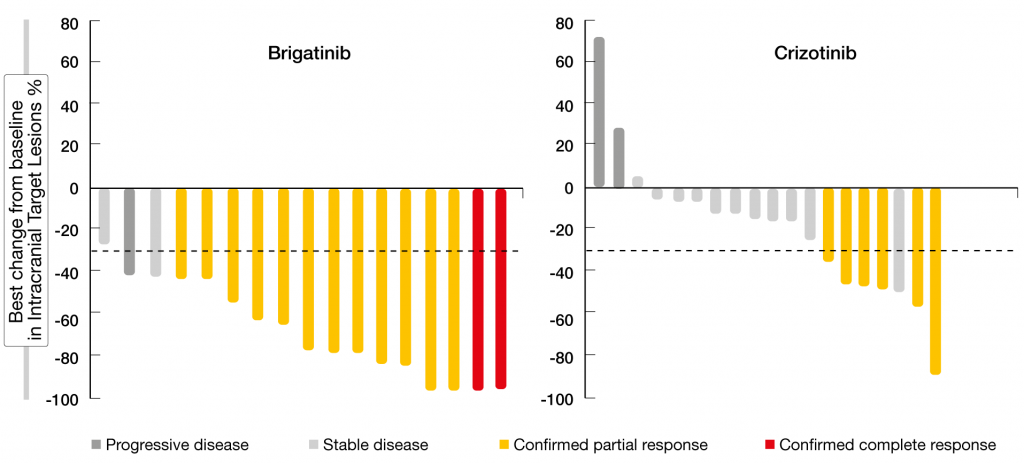
The randomized, phase III ALTA-1L trial tested the next-generation ALK/ROS1 inhibitor brigatinib against the first-generation TKI crizotinib in patients with advanced ALK-positive NSCLC who were ALK-inhibitor–naïve. One line of prior systemic therapy in the advanced setting was allowed. Brigatinib was administered at a dose of 180 mg/day after a 7-day lead-in at 90 mg (n = 137). In the control arm, 138 patients received crizotinib 250 mg BID. Califano et al. presented the first interim analysis at ELCC 2019 [11]. PFS according to blinded independent review committee, which was defined as the primary endpoint, was highly in favor of brigatinib (not reached vs. 9.8 months; HR, 0.49; p = 0.0007). At one year, 67 % vs. 43 % of patients were progression-free. The subgroup analysis demonstrated consistent PFS benefits with brigatinib across all subgroups. ORR was numerically higher for brigatinib compared to crizotinib (71 % vs. 60 %); median duration of response had not been reached in the experimental arm and was 11.1 months in the control arm. In patients with measurable brain metastases at baseline, confirmed intracranial responses occurred in 78 % vs. 29 % (OR, 10.42; p = 0.0028). In those with any brain lesions at baseline, confirmed intracranial ORR was 67 % vs. 17 % (OR, 13.00; p < 0.0001). All patients treated with brigatinib achieved shrinkage of measurable CNS metastases, which did not apply to the crizotinib-treated cohort (Figure). Accordingly, intracranial PFS was significantly improved in the ITT population (HR, 0.42; p = 0.0006) and in patients with any brain metastases at baseline (not reached vs. 5.6 months; HR, 0.27; p < 0.0001). For those without baseline brain metastases, results for intracranial PFS are still immature.
Figure: ALTA-1L: intracranial best target lesion responses in patients with measurable brain disease
Significant delay of CNS progression
According to a competing risk analysis, brigatinib induced significant improvements of the time to intracranial CNS progression without prior systemic progression (cause-specific HR for CNS progression, 0.30; p < 0.001) and the time to systemic progression without prior intracranial CNS progression (cause-specific HR for systemic progression, 0.51; p = 0.017). Thus, brigatinib significantly delayed both CNS and systemic progression compared to crizotinib. While excess AEs observed with crizotinib were dominated by gastrointestinal symptoms, transaminitis, bradycardia, edema, and visual effects, brigatinib-associated excess AEs comprised mainly asymptomatic increases of CPK, lipase, and amylase. Dose reductions were largely protocol-mandated due to these laboratory abnormalities. No clinical cases of pancreatitis occurred in either arm. Early-onset interstitial lung disease/pneumonitis within 2 weeks of treatment initiation appears to be unique to brigatinib among the ALK TKIs, but was rare at 3 %, with a lower event rate compared to later-line trials [12]. Based on this data, brigatinib was shown to be a promising first-line treatment option for ALK-positive advanced NSCLC.
Treatment duration with brigatinib
The international Expanded Access Program (EAP) for brigatinib that was opened in July 2016 includes patients with ALK-positive, locally advanced or metastatic NSCLC who have exhausted available therapies or are unable to participate in a clinical study. Between July 2016 and November 2018, 604 patients in 21 countries across Western Europe, the Asian-Pacific region, and South America entered the EAP. They received brigatinib across all treatment lines. The analysis of patient outcomes presented at ELCC was conducted with the objective to evaluate the real-world activity of brigatinib in ALK-rearranged NSCLC [13]. As no clinical outcome endpoints have been defined in the EAP, time to treatment discontinuation, which is highly correlated to PFS particularly in TKIs, was used as a proxy for the tolerability and efficacy of treatment. Patients included in the analysis were resistant or intolerant to ≥ 1 prior ALK TKI. In the majority of cases (67.2 %), brigatinib was administered in the third or later lines. Among ALK TKIs, ceritinib, crizotinib and alectinib had been used most frequently prior to brigatinib. Despite this heterogeneity, median time to discontinuation amounted to almost one year (10.95 months) across all lines of treatment. The probability of continued use at 6 and 12 months was 67.1 % and 48.6 %, respectively. When analyzed by type of prior ALK TKI therapy, continuous use of brigatinib was seen after alectinib (n = 111; median time to discontinuation of brigatinib, 8.72 months) and ceritinib (n = 249; median time to discontinuation, 10.33 months). Brigatinib was also used post-lorlatinib, with a median time to discontinuation of 7.5 months. Both the probability of continued use of brigatinib and the median time to brigatinib discontinuation decreased with increasing prior ALK TKI treatment lines. A total of 260 patients discontinued treatment. Only 4 of these (0.7 %) reported discontinuation due to AEs. The main reason for brigatinib treatment discontinuation was lack of efficacy (9.6 %). Overall, the treatment duration observed for brigatinib in a real-world setting was encouraging irrespective of prior ALK inhibitor treatment, and the safety profile proved manageable.
REFERENCES
- Vaishnavi A et al., TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov 2015; 5(1): 25-34
- Stransky N et al., The landscape of kinase fusions in cancer. Nat Commun 2014; 5: 4846
- Drilon A et al., Activity of larotrectinib in TRK fusion lung cancer. ELCC 2019, abstract 1110
- Paz-Ares L et al., Entrectinib in NTRK fusion-positive non-small cell lung cancer (NSCLC): integrated analysis of patients enrolled in STARTRK-2, STARTRK-1 and ALK-372-001. ELCC 2019, abstract 1130
- Patil T et al., The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol 2018; 13(11): 1717-1726
- Ardini E et al., Entrectinib, a Pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther 2016; 15(4): 628-639
- Drilon A et al., Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov 2017; 7(4): 400-409
- Barlesi F et al., Entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small cell lung cancer (NSCLC): integrated analysis of STARTRK-2, STARTRK-1 and ALKA-372-001. ELCC 2019, abstract 1090
- Shaw AT et al., Crizotinib in advanced ROS1-rearranged non-small cell lung cancer (NSCLC): overall survival (OS) and updated safety from PROFILE 1001. ELCC 2019, abstract 1070
- Shaw AT et al., Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014; 371(21): 1963-1971
- Califano R et al., Brigatinib vs crizotinib in the phase 3 ALTA-1L trial, ELCC 2019, abstract 1060
- Kim DW et al., Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol 2017; 35(22): 2490-2498
- Lin HM et al., Treatment duration of brigatinib in patients enrolled in the international Expanded Access Program (EAP). ELCC 2019, abstract 1080