Immunotherapy: analyses elucidating durvalumab & pembrolizumab activity
PACIFIC: OS after subsequent immunotherapies
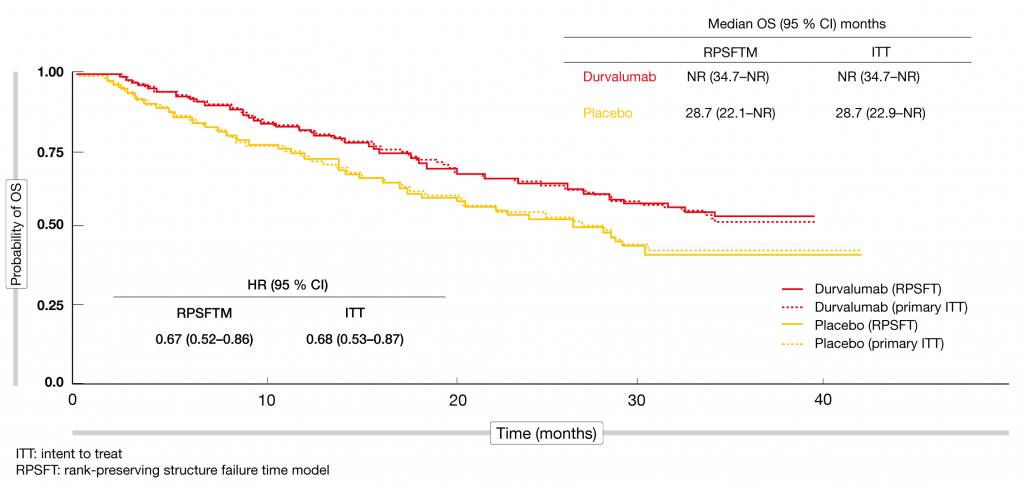
The phase III, randomized, double-blind, international PACIFIC trial established durvalumab in the treatment of patients with stage III, unresectable NSCLC without progression after definitive platinum-based concurrent chemoradiotherapy. Within 6 weeks of chemoradiation, patients were randomized to either durvalumab 10 mg/kg every 2 weeks (Q2W) for up to 12 months (n = 476) or matching placebo (n = 237). PFS and OS were defined as the primary endpoints. Durvalumab provided unprecedented clinical improvement for both outcomes. At the time of the primary analysis, median OS had not been reached in the experimental arm and was 28.7 months in the control arm (HR, 0.68; p = 0.0025) [1, 2]. The survival curves separated early on and remained separated despite the limited treatment duration, which suggests long-term benefit of the PD-L1 inhibitor. Ouwens et al. explored the question of whether subsequent immunotherapy influenced OS findings in the PACIFIC trial [3]. In the durvalumab and placebo arms, 8 % and 22 % of patients, respectively, received immunotherapy after discontinuation of the study treatment. Other anticancer treatments were administered in 33 % and 32 %, respectively. No subsequent therapy was given in 59 % and 46 %, respectively. The rank-preserving structure failure time model (RPSFTM) was used to adjust OS for subsequent immunotherapy in PACIFIC. After statistical removal of subsequent immunotherapy, the OS results were consistent with the intent-to-treat (ITT) analysis. The curves were virtually superimposable, and the HRs for the differences between durvalumab and placebo varied only by 0.1 (0.67 and 0.68 for RPSFTM and ITT, respectively; Figure 1). Overall, this exploratory analysis of the PACIFIC trial added to the robustness of the data demonstrating durvalumab activity in patients with unresectable, stage III NSCLC.
Figure 1: Overall survival adjusted for subsequent immunotherapy in the PACIFIC trial: superimposable curves for durvalumab and placebo according to the ITT and RPSFT analyses
Effect of PD-L1 expression on PROs
In the PACIFIC study, durvalumab treatment did not cause deterioration of patient symptoms, functioning or global health/quality of life compared to placebo in the ITT population [4]. A retrospective analysis presented at ELCC 2019 investigated the impact of tumor PD-L1 expression on patient-reported outcomes (PROs) to improve the understanding of the benefit/risk profile of durvalumab across all PD-L1 subgroups [5]. PROs (i.e., symptoms, functioning and global health status/quality of life) were assessed using the EORTC QLQ-C30 and QLQ-LC13 questionnaires. A clinically meaningful difference in PROs was defined as a 10-point change in score. Of 713 randomized patients, 451 (63 %) were evaluable for PD-L1 status; among these, 303 (67.2 %) had PD-L1 expression ≥ 1 %, while 148 (32.8 %) were PD-L1–negative. In 262 patients (37 %), the PD-L1 status was unknown. Similar to the ITT population, the majority of PROs remained stable over time from baseline across all PD-L1 subgroups including those with unknown PD-L1 status, with no clinically meaningful differences for durvalumab vs. placebo. The consistency of results for the PD-L1 subgroups with those of the ITT population suggests that symptoms, functioning and global health status/quality of life were maintained regardless of PD-L1 expression. The authors concluded that these data further support the use of durvalumab after concomitant chemoradiation as the standard of care.
MYSTIC: adjusted survival
The global, randomized, open-label phase III MYSTIC trial evaluated first-line durvalumab (n = 374), durvalumab plus tremelimumab (n = 372), and platinum-based chemotherapy (n = 372) in patients with metastatic NSCLC irrespective of their PD-L1 expression status. The primary endpoints were assessed in patients with PD-L1 expression ≥ 25 % and included PFS for the combination vs. chemotherapy, OS for durvalumab vs. chemotherapy, and OS for the combination vs. chemotherapy. Even though the OS difference for durvalumab vs. chemotherapy did not reach statistical significance due to multiple testing, it was clinically meaningful, with an HR of 0.76 (16.3 vs. 12.9 months; p = 0.036) [6]. At two years, 38.3 % vs. 22.7 % of patients were alive. Likewise, durvalumab plus tremelimumab did not induce a significant OS benefit over chemotherapy (11.9 vs. 12.9 months; HR, 0.85), although the 2-year OS rates were in favor of the combination (35.4 % vs. 22.7 %). Reinmuth et al. explored the effect of subsequent immunotherapy on the OS outcome with durvalumab compared to chemotherapy in the population with PD-L1 expression ≥ 25 % enrolled in MYSTIC [7]. Subsequent immunotherapy had been administered in 14 % and 67 % of durvalumab- and chemotherapy-treated patients, respectively. Using the 2-stage model to adjust for these imbalances, the investigators found that the OS benefit of durvalumab over chemotherapy was even more pronounced than in the primary analysis, with an HR of 0.66 (median OS, 16.2 vs. 11.5 months; p = 0.002). Thus, this exploratory analysis suggests that the high rate of subsequent immunotherapy in the control arm confounded the primary OS findings obtained in the MYSTIC study.
Outcomes according to patient characteristics
Another analysis of the MYSTIC trial presented at ELCC related to the treatment efficacy in clinically relevant patient subgroups (i.e., sex, age, percentage of tumor-associated immune cells expressing PD-L1 ≥ 25 % vs. < 25 %, histology, smoking history, ethnicity, ECOG performance status) [8]. Patients with a tumor cell PD-L1 expression ≥ 25 % were included in the OS analyses, which consistently showed favorable HRs for durvalumab versus chemotherapy in the defined subgroups. This was in keeping with the primary analysis. For durvalumab plus tremelimumab, the HRs compared to chemotherapy were less favorable across the subgroups. The interpretation of the findings according to immune cell PD-L1 expression was limited due to the restriction to patients with tumor cell PD-L1 expression ≥ 25 % and small sample sizes; here, further investigation is required. Also, a safety analysis that specifically evaluated higher-grade treatment-related AEs revealed higher rates of these AEs with chemotherapy than with durvalumab or the combination. AEs leading to discontinuation of treatment were slightly more common with durvalumab plus tremelimumab than with durvalumab alone or chemotherapy. Any-grade immune-mediated AEs occurred more frequently with the combination than with durvalumab monotherapy (28.3 % vs. 13.6 %). The authors noted that the safety profile of durvalumab monotherapy and durvalumab plus tremelimumab was manageable and in line with previous results.
Final analysis of KEYNOTE-042
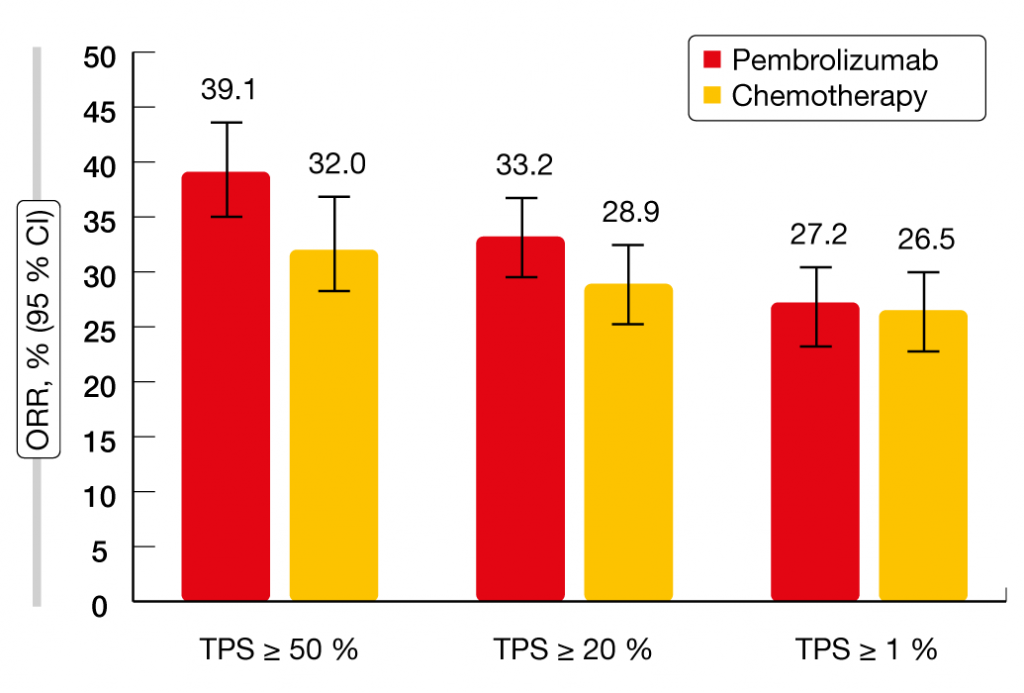
Mok et al. reported the final analysis of the KEYNOTE-042 study that tested pembrolizumab vs. platinum-based chemotherapy in patients with PD-L1–expressing locally advanced or metastatic NSCLC [9]. Both treatment arms contained 637 patients. The primary analysis had revealed OS improvement with pembrolizumab 200 mg Q3W for up to 35 cycles compared to carboplatin plus paclitaxel or carboplatin plus pemetrexed for up to 6 cycles at all prespecified PD-L1 cut points (i.e., tumor proportion score [TPS] ≥ 50 %, ≥ 20 %, ≥ 1 %) [10]. After an additional follow-up of 6 months, pembrolizumab treatment continued to confer significant OS improvement over chemotherapy independent of PD-L1 expression. Median OS was longer in patients with PD-L1 TPS ≥ 50 % (20.0 vs. 12.2 months; HR, 0.70), ≥ 20 % (18.0 vs. 13.0 months; HR, 0.77), and ≥ 1 % (16.4 vs. 12.1 months; HR, 0.82). However, the analysis of patients with TPS 1 % to 49 % showed no statistically significant OS benefit (13.4 vs. 12.1 months; HR, 0.91), which implies that the benefit was mostly driven by the group with the highest PD-L1 expression. No PFS improvement was seen with pembrolizumab vs. chemotherapy for any PD-L1 expression subgroup. In terms of response rates, the results suggested a pembrolizumab-associated advantage in patients with high PD-L1 expression (Figure 2). Duration of response was longer in the experimental arm compared to the control arm across all PD-L1 expression subgroups. This means that once patients benefit from treatment, a durable response of approximately 20 months can be expected irrespective of PD-L1 status. No new safety signals were identified. Overall, these results support the first-line use of pembrolizumab monotherapy in patients with PD-L1–expressing NSCLC.
Figure 2: Response rates observed in KEYNOTE-042 according to PD-L1 expression
Pooled KEYNOTE data in the elderly
In view of the fact that patients aged ≥ 75 years are generally underrepresented in clinical studies, Nosaki et al. conducted a pooled analysis of the efficacy and safety of pembrolizumab in elderly patients included in the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 trials [11]. KEYNOTE-010 had assessed pembrolizumab 2 mg/kg or 10 mg/kg Q3W versus docetaxel in previously treated advanced NSCLC [12], while both KEYNOTE-024 [13] and KEYNOTE-042 [10] had evaluated pembrolizumab 200 mg Q3W compared to platinum-based chemotherapy in the first-line setting, with the required PD-L1 TPS defined at ≥ 50 % and ≥ 1 % in KEYNOTE-024 and KEYNOTE-042, respectively. Overall, 149 patients aged ≥ 75 years had received pembrolizumab in these studies, while 115 had been treated with chemotherapy. Median age was 77 years in both arms. Among younger patients, pembrolizumab and chemotherapy had been administered in 1,332 and 1,016 individuals, respectively. Older patients showed clinically relevant OS improvements with pembrolizumab compared to chemotherapy in both treatment-naïve and previously treated settings. The OS benefit observed for the PD-L1 TPS groups ≥ 1 % and ≥ 50 % was consistent with the benefits seen in other populations included in the three studies and among the younger patients enrolled in these trials. In the group with PD-L1 TPS ≥ 1 %, both patients aged ≥ 75 and < 75 years derived a 24 % reduction in mortality risk with pembrolizumab treatment (HR, 0.76). For those with PD-L1 TPS ≥ 50 %, HRs were 0.40 and 0.67 for the older and younger groups, respectively. Elderly patients also fared better according to the analysis of the treatment-naïve, highly PD-L1–expressing population (i.e., TPS ≥ 50 %); this revealed HRs of 0.41 and 0.71 for the older and younger patients, respectively. Pembrolizumab showed a comparable and favorable safety profile independent of age. Among the older patients, there were no increases in toxicity, and the majority of immune-mediated AEs and infusion reactions were grade 1/2. No grade 5 immune-mediated AEs occurred in this cohort. Median treatment duration with pembrolizumab was even longer in patients aged ≥ 75 years than in those aged < 75 years (5.6 vs. 4.3 months, respectively).
REFERENCES
- Antonia SJ et al., Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377(20): 1919-1929
- Antonia SJ et al., Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379(24): 2342-2350
- Ouwens M et al., Impact of subsequent post-discontinuation immunotherapy on overall survival in patients with unresectable, stage III NSCLC from PACIFIC. ELCC 2019, abstract 830
- Hui R et al., Patient-reported outcomes with durvalumab after chemoradiation in locally advanced, unresectable NSCLC: data from PACIFIC. WCLC 2018, abstract PL02.02
- Garassino MC et al., Patient-reported outcomes with durvalumab by PD-L1 expression in unresectable, stage III NSCLC (PACIFIC). ELCC 2019, abstract LBA2
- Rizvi N et al., Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. ESMO I-O 2018, abstract LBA6
- Reinmuth N et al., Effect of post-study immunotherapy on overall survival outcome in patients with metastatic NSCLC treated with first-line durvalumab vs chemotherapy in the phase 3 MYSTIC study. ELCC 2019, abstract LBA4
- Cho CB et al., Efficacy and safety of first-line durvalumab ± tremelimumab vs platinum-based chemotherapy based on clinical characteristics in patients with metastatic NSCLC: results from MYSTIC. ELCC 2019, abstract LBA3
- Mok TS et al., Final analysis of the phase 3 KEYNOTE-042 study: pembrolizumab vs. platinum-based chemotherapy as first-line therapy for patients with PD-L1-positive locally advanced or metastatic NSCLC. ELCC 2019, abstract 1020
- Mok TSK et al., Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019 Apr 4 pii: S0140-6736(18)32409-7.
- Nosaki K et al., Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1 positive advanced NSCLC: pooled analysis from KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042. ELCC 2019, abstract 1030_PR
- Herbst RS et al., Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387(10027): 1540-1550
- Reck M et al., Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med 2016; 375: 1823-1833