Ensartinib outperforms crizotinib as a treatment for patients with ALK-positive NSCLC who were naïve to ALK-targeted therapy
eXalt3 was a randomized, open-label, comparator-controlled phase III trial designed to evaluate the efficacy and safety of treatment with either of two anaplastic lymphoma kinase (ALK) inhibitors alone, ensartinib or crizotinib, in the treatment of advanced or metastatic ALK-positive non-small cell lung cancer (NSCLC) patients. The trial enrolled patients with an ALK-positive, stage IIIB or IV NSCLC confirmed either by local FDA-approved assays or central confirmation via fluorescence in situ hybridization (FISH). Although patients with previous ALK inhibitor treatment were excluded, patients with ECOG performance status of 0 to 2 with ≤1 prior chemotherapy regimen were allowed. Patients were stratified according to prior chemotherapy status, ECOG performance status, geographical location, and brain metastases at baseline.
A total of 290 patients were randomized 1-to-1 to receive either ensartinib daily or crizotinib twice per day until disease progression. No crossover between study arms was allowed during the trial. The primary endpoint was blinded and an independent review committee (BIRC) assessed median progression-free survival (mPFS) in the Intention-to-treat (ITT) population.
The baseline characteristics were similar in both treatment groups with around one-third of patients presenting brain metastases at baseline (33% vs 39%) or having prior chemotherapy regimen (24% vs 29%), and a higher proportion of never smokers (59% vs 64%) as compared to current or former smokers (41% vs 36%).
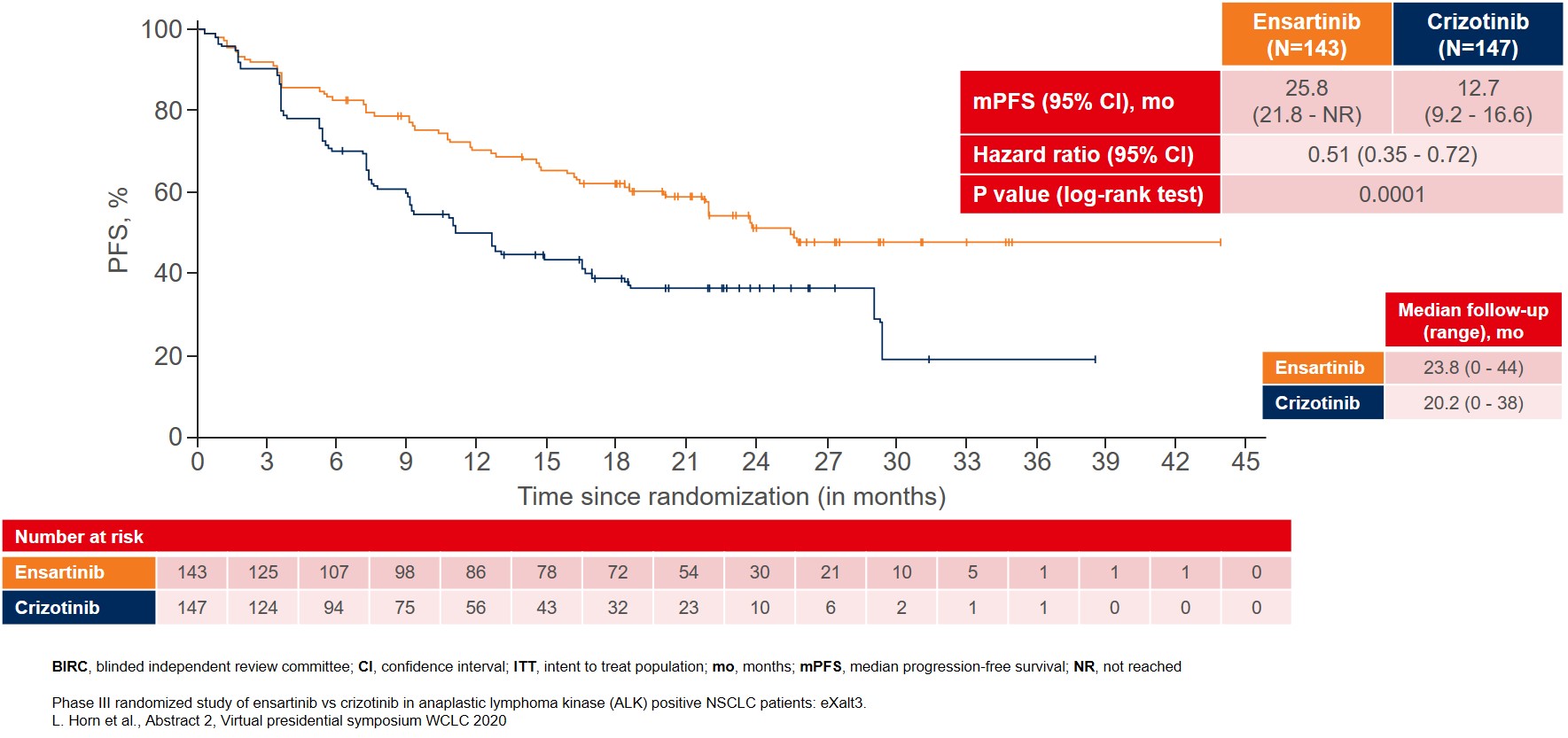
The interim analysis showed that the primary endpoint was met with BIRC-assessed mPFS for patients treated with ensartinib where mPFS improved to 25.8 months as compared to 12.7 months for patients treated with crizotinib (Figure 1).
Figure 1: BIRC-assessed median PFS in the ITT population as the primary endpoint of the eXalt3 study
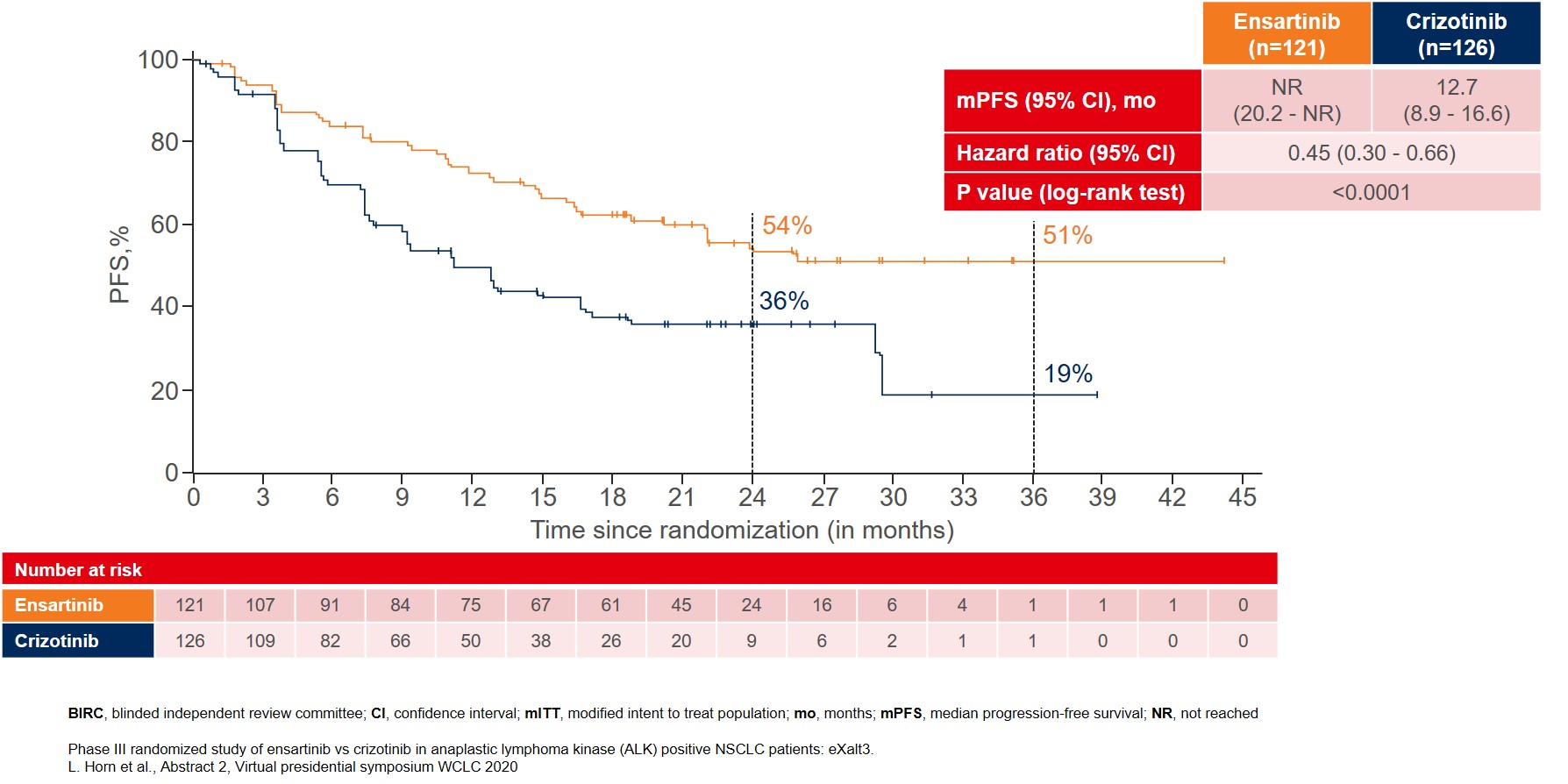
More remarkable improvement in mPFS was reported in the modified ITT (mITT) population, which included patients with centrally confirmed ALK gene rearrangements by FISH testing. In this mITT population (n=247), mPFS was not reached (NR) for ensartinib as compared to 12.7 months for crizotinib (Figure 2).
Figure 2: BIRC-assessed median PFS in the modified ITT population with patients that were centrally confirmed for ALK gene rearrangements by FISH testing
Other efficacy endpoints also showed improvement, e.g. objective response rate (ORR) (75% vs 67%) and duration of response (DOR) (NR vs 27.3 months), in favor of ensartinib as compared to crizotinib. The overall survival (OS) data were still immature, with the median OS not reached for either of the treatment arms, with around 20% of events documented at the time of analysis in both treatment arms. Sub-group analysis of patients without brain metastases at baseline (n=157) in the mITT population showed that ensartinib significantly delayed time to treatment failure (TTF) as compared to crizotinib (4.2 vs 23.9 months; HR 0.32, 95% CI 0.15-0.64; p = 0.0011).
The safety profile for ensartinib was reported to be as tolerable as crizotinib with similar rates of serious treatment-related adverse events (TRAEs) (8% vs 6%), TRAEs leading to dose reduction (24% vs 20%) and TRAEs leading to drug discontinuation (9% vs 7%). The most common TRAEs for patients on ensartinib were skin toxicities including rash and pruritis as well as elevation of liver transaminases, most of which were grade 1 or 2. The most common TRAEs for the crizotinib group were consistent with previous studies such as elevation of liver transaminases, gastrointestinal toxicities and edema.
With longer follow-up, ensartinib shows a positive trend towards improved mPFS, representing a new first-line treatment option for patients with ALK-positive NSCLC.
More posts
KRAS, HER2 & ALK: targeted options and sequencing issues
The KRAS p.G12C mutation is a key oncogenic driver occurring in approximately 13 % of lung adenocarcinomas and is associated with poor patient outcomes. The first-in-class, highly selective and irreversible KRASG12C inhibitor sotorasib has shown durable clinical benefit in a cohort of 59 heavily pretreated patients with NSCLC included in phase I of the CodeBreaK 100 study.
Pushing the bounds in early-stage lung cancer
Approximately 30 % of patients with non-small-cell lung cancer (NSCLC) present with resectable disease at diagnosis. Surgery with curative intent is the recommended treatment here, followed by adjuvant cisplatin-based chemotherapy in stage II/IIIA and select cases of stage IB disease. However, recurrence rates remain high across disease stages, regardless of postoperative chemotherapy use.
Preface
The 2020 World Conference of Lung Cancer (WCLC) originally scheduled for August 2020 in Singapore had to be postponed to January 2021 due to the COVID-19 pandemic and was finally held as a worldwide virtual conference from 28th to 31st January. WCLC, which is the leading gathering of international scientists, researchers and patient advocates in the field of lung cancer and thoracic malignancies, continues to provide a forum to connect, share knowledge and learn about the latest developments in the research and treatment of these diseases.