EGFR-targeted options in a changing treatment landscape
Amivantamab plus lazertinib
The combination of amivantamab, a bispecific antibody that targets both EGFR and MET, and the potent third-generation EGFR TKI lazertinib is being explored in patients with advanced NSCLC. Amivantamab has demonstrated clinical activity across various types of EGFR-mutant NSCLC harboring both activating and resistance mutations [1] and was granted FDA Breakthrough Therapy Designation for EGFR-mutant NSCLC with exon 20 insertion after progression on platinum-based chemotherapy. Also, lazertinib was shown to be efficacious in NSCLC patients with activating EGFR mutations, T790M resistance mutation, and CNS disease [2, 3]. As with other third-generation EGFR inhibitors, rates of EGFR-related toxicity such as rash and diarrhea are low with lazertinib treatment. It was hypothesized that the combination of these agents might have the potential to delay or prevent the emergence of resistance without increasing toxicity.
Patients with metastatic or unresectable EGFR-mutant (i.e., exon 19 deletions or L858R mutations) NSCLC were treated with amivantamab plus lazertinib in the phase I CHRYSALIS study [4]. The recommended phase II dose was found to be equivalent to the recommended monotherapy doses of each agent: amivantamab 1,050 mg in patients with body weight < 80 kg or 1,400 mg in patients weighing ≥ 80 kg, and lazertinib 240 mg. Amivantamab is administered intravenously once weekly in cycle 1 and two-weekly in subsequent cycles, while lazertinib is taken orally once daily. The dose escalation and dose expansion cohorts contained 26 and 65 patients, respectively; in the dose expansion cohort, 45 were osimertinib-resistant and chemotherapy-naïve, while 20 were treatment-naïve. Within the total group of 91 patients, 37 % had brain metastases at baseline. The number of prior treatment lines ranged from 0 to 9. Fifty-nine percent had received first- or second-generation EGFR TKIs, and 58 % had been treated with third-generation TKIs.
Rapid and lasting responses
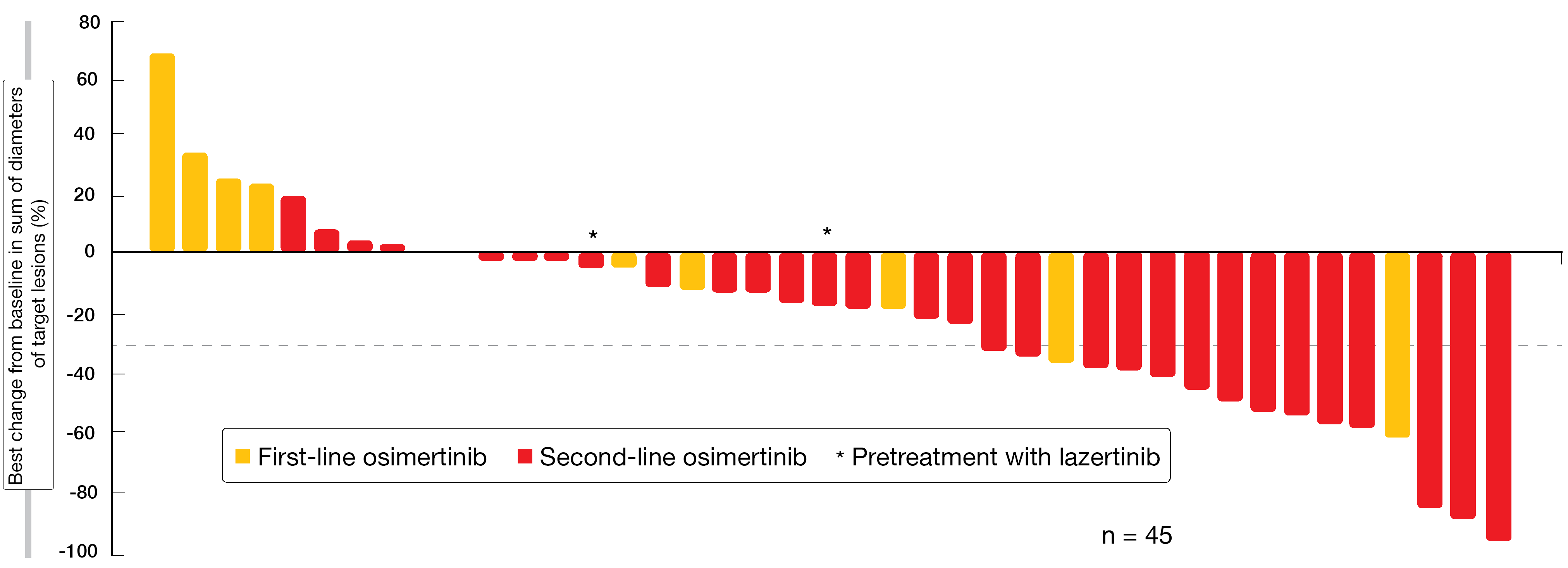
In the osimertinib-resistant, chemotherapy-naïve population, 36 % of patients obtained ORR, with one and 15 patients experiencing complete and partial remission, respectively. The clinical benefit rate was 60 %. Tumor regressions were observed regardless of the line of pretreatment with osimertinib and also occurred in patients who had progressed on prior lazertinib (Figure 1). Responses are ongoing in the majority of cases. Biomarker and CNS analyses for this group will be presented at future meetings. In the treatment-naïve group, ORR and clinical benefit rates were 100 % each. Patients showed deep responses regardless of the EGFR mutation genotypes. Time to first response was short at a median of 1.5 months. After a median follow-up of 7 months, the treatment is ongoing in all patients.
The combination of amivantamab and lazertinib was safe and well tolerated. No dose-limiting toxicity occurred during escalation. AEs were predominantly grade 1 and 2; treatment-related serious AEs and grade ≥ 3 AEs were observed in 6 % and 11 %, respectively. As expected, the most common event was skin rash, which occurred in 85 %, followed by infusion-related reactions (65 %). Infusion-related reactions were mostly observed during the first administration and did not give rise to treatment discontinuations or dose modifications. In 19 % each, AEs led to dose interruption or reduction of either one or both drugs. However, discontinuation of either one or both drugs only became necessary in 6 %. The rates of AEs were similar across the dose-escalation, treatment-naïve, and osimertinib-resistant/chemotherapy-naïve groups.
According to the authors’ conclusions, amivantamab can be safely combined with lazertinib and the combination is active in patients with advanced EGFR-mutated NSCLC. An analysis of the efficacy by mechanism of resistance is ongoing. New studies assessing amivantamab plus lazertinib have been started, including the phase III MARIPOSA trial (NCT04487080) that is comparing frontline use of the combination with osimertinib.
Figure 1: Changes in target lesions with amivantamab plus lazertinib in osimertinib-resistant, chemotherapy-naïve patients
Antiangiogenic combination partner: apatinib
Blockage of the vascular endothelial growth factor receptor (VEGFR) pathways was demonstrated to enhance the efficacy of EGFR TKI treatment [5]. Therefore, the multicenter, randomized, double-blind, placebo-controlled, phase III ACTIVE trial evaluated the combination of the EGFR TKI gefitinib and the oral small molecule VEGFR2 TKI apatinib as first-line treatment in advanced lung cancer [6]. At 30 sites in China, chemotherapy-naïve patients with locally advanced, metastatic or recurrent non-squamous, EGFR-positive NSCLC were randomized to either apatinib 500 mg daily plus gefitinib 250 mg daily (n = 157) or placebo plus gefitinib (n = 156).
PFS according to independent radiology review committee, which constituted the primary endpoint, was significantly in favor of the combination (13.7 vs. 10.2 months; HR, 0.71; p = 0.0189; Figure 2). Almost all subgroups benefited from the addition of the VEGFR2 TKI. The study arms did not differ with respect to ORR (77.1 % vs. 73.7 %) or DCR (84.7 % vs. 87.8 %), although apatinib plus gefitinib significantly improved depth of response ≥ 30 % (89.2 % vs. 79.5 %; p = 0.0209) and depth of response ≥ 50 % (64.3 % vs. 52.6 %; p = 0.0238). Also, duration of response was longer in the experimental arm (12.9 vs. 9.3 months; HR, 0.64; p = 0.005). Apatinib plus gefitinib was generally well tolerated, with manageable toxicity. Dose interruptions of any drug due to treatment-emergent AEs (TEAEs) occurred in 59.9 % vs. 22.7 %, and dose reductions became necessary in 48.4 % vs. 4.5 %. However, only 5.1 % in the combination arm discontinued treatment due to TEAEs (vs. 3.2 % in the control arm). No unexpected safety signals were identified beyond the established safety profile of each agent.
A biomarker analysis conducted in 145 patients (73 and 72 in the experimental and control arms, respectively) showed that patients with TP53 exon 8 mutation derived greater benefit from apatinib plus gefitinib (HR, 0.24) than those with TP53 non-exon 8 mutation (HR, 0.79). Patients without TP53 mutation did not experience any PFS prolongation (HR, 0.92). However, due to the small sample size, this observation requires confirmation in a large study. Similar PFS benefits were seen with EGFR exon 19 deletion and exon 21 L858R mutation (HRs, 0.67 and 0.72, respectively). The resistance biomarker analysis revealed that patients in both study arms developed a similar T790M resistance pattern, with T790M positivity in 37.8 % and 37.0 %, respectively. In their summary, the authors stated that apatinib plus gefitinib might become a new first-line option for advanced EGFR-mutant NSCLC. This dual oral regimen provides convenient treatment for patients who require long-term therapy.
Figure 2: Superior progression-free survival with apatinib plus gefitinib compared to placebo plus gefitinib
No benefit of osimertinib plus bevacizumab
Less favorable results were generated for the combination of the third-generation EGFR TKI osimertinib, which is the standard option in T790-mutant NSCLC, with the anti-VEGF antibody bevacizumab. The randomized phase II WJOG8715L study compared osimertinib 80 mg daily plus bevacizumab 15 mg/kg Q3W (n = 40) with osimertinib 80 mg daily (n = 41) in patients with advanced, EGFR-TKI-resistant adenocarcinoma of the lung that had acquired the T790M mutation.
According to the primary analysis of the trial presented at ESMO 2020, the combination failed to improve PFS, which was defined as the primary endpoint (9.4 vs. 13.5 months; HR, 1.44; p = 0.20) [7]. This lack of efficacy was confirmed by the results of the subgroup analysis. In patients who had received prior anti-VEGF therapy, PFS was even shorter for the osimertinib plus bevacizumab combination than for the other regimens (osimertinib plus bevacizumab without anti-VEGF pretreatment, osimertinib monotherapy with and without anti-VEGF history). The ORR was higher in the combination arm than in the osimertinib monotherapy arm (71.8 % vs. 55.0 %), although no significant differences between the study arms were noted for time to treatment failure or OS. At the same time, AEs such as proteinuria and hypertension occurred significantly more frequently in the experimental arm.
Antibody drug conjugate patritumab deruxtecan
HER3 is expressed in approximately 80 % of EGFR-mutated lung cancers, and overexpression has been linked to worse clinical outcomes [8]. The investigational HER3-directed antibody drug conjugate patritumab deruxtecan (U3-1402) was tested in patients with metastatic or unresectable EGFR-mutated NSCLC in a phase I study. The dose-escalation part included 12 patients who had progressed on osimertinib or were T790M-negative after progression on erlotinib, gefitinib, or afatinib. In the dose-expansion cohort (n = 45), patients after ≥ 1 EGFR TKI and ≥ 1 platinum-based chemotherapy were treated. Both cohorts received patritumab deruxtecan at the recommended phase II dose of 5.6 mg/kg Q3W.
Yu et al. presented updated findings of the combined cohorts [9]. This was a heavily pretreated population with a median of 4 prior lines of therapy. Eighty-six percent had received osimertinib prior to study inclusion. Platinum-based chemotherapy had been administered in 90 %, and anti-PD-(L)-1 agents in 40 %. A history of CNS metastases was present in 47 %, as asymptomatic stable brain lesions were allowed. Patients were not selected for HER3 expression, although tumor tissue was collected prior to the initiation of study treatment for a retrospective analysis. This showed that the majority of patients had evidence of HER3 expression.
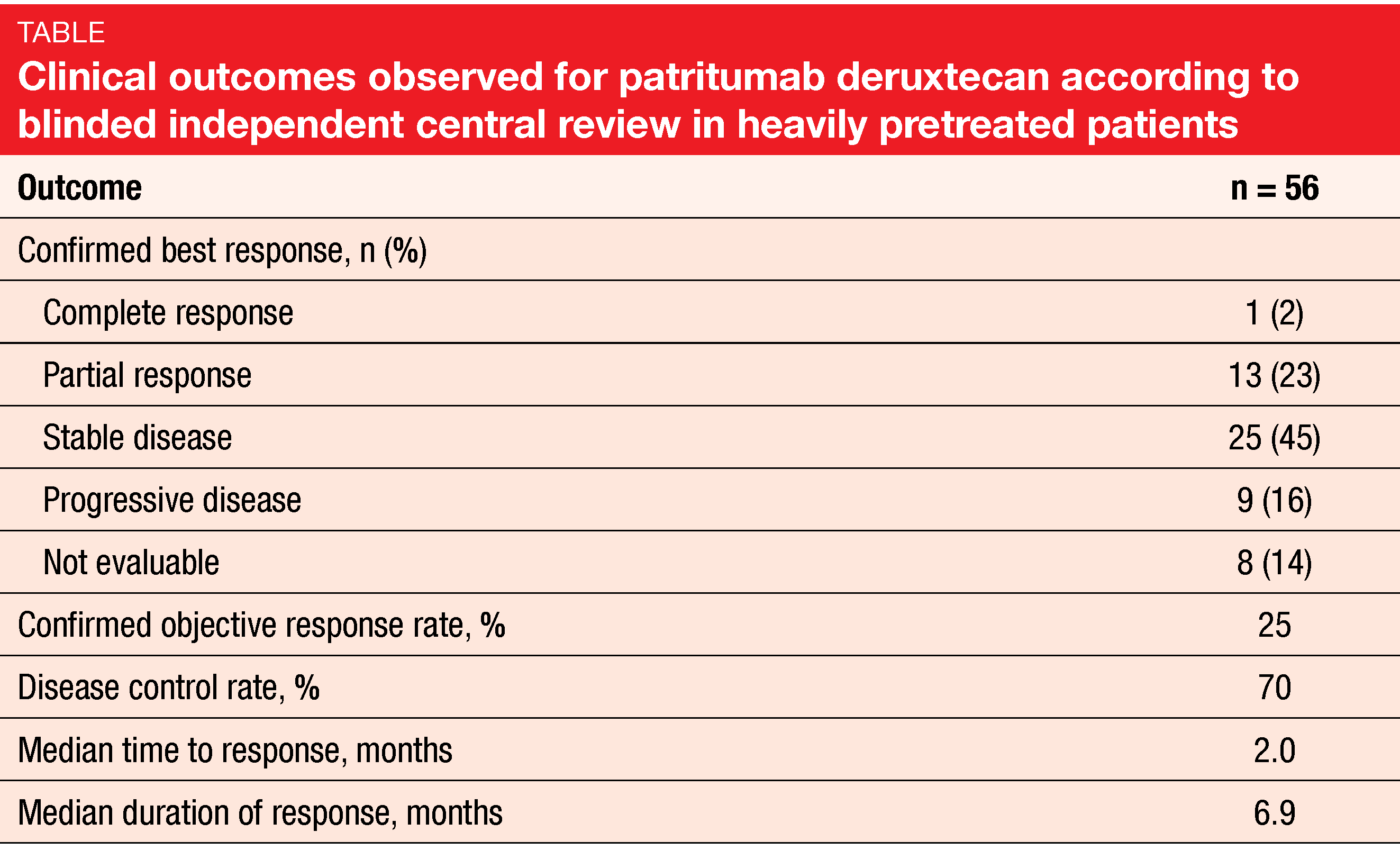
Fifty-six patients were evaluable for response. After a median follow-up of 5 months, the confirmed ORR was 25 %, which did not include 3 partial remissions yet to be confirmed (Table). One patient (2 %) achieved complete remission. Disease control resulted in 70 %. Responses emerged early on, and most patients experienced some degree of tumor shrinkage. According to next-generation sequencing on plasma or tumor tissue, clinically meaningful anti-tumor activity emerged in patients with diverse resistance mechanisms; this included confirmed partial responses in patients with EGFR C797S mutation, MET amplification, HER2 amplification, BRAF fusion, and PIK3CA mutation. Median PFS was not mature yet.
Patritumab deruxtecan 5.6 mg/kg continued to demonstrate a manageable safety profile. The most common grade ≥ 3 TEAEs were thrombocytopenia and neutropenia, although no patients discontinued treatment due to these toxicities. Three cases (5.3 %) of interstitial lung disease were reported. Most TEAEs responded well to dose reduction and interruption. Collectively, these data support further clinical investigation of patritumab deruxtecan in a patient population with no available targeted therapy options. A phase II study of single-agent patritumab deruxtecan in patients after failure of EGFR TKIs and platinum-based chemotherapy is planned to start in early 2021.
Evaluation of low-dose afatinib
Although afatinib is an effective treatment option for patients with EGFR-positive NSCLC, its toxicities, particularly nail and skin AEs as well as diarrhea, often require dose modifications. Based on the assumption that a lower dose from the initiation of treatment might contribute to improving efficacy and safety, Noro et al. conducted a multicenter, single-arm, open-label, phase II trial assessing afatinib 20 mg daily in treatment-naïve patients with advanced NSCLC harboring common EGFR mutations [10]. Patients who showed complete or partial response or disease stabilization without tumor growth at 8 weeks continued the 20 mg dose, while in those with growing tumors, the dose was escalated to 30 mg or 40 mg daily. When drug-related grade ≥ 2 AEs occurred after dose increases, reductions were performed in 10-mg increments. Afatinib plasma concentrations were measured on day 9 after the start of treatment and at the time of disease progression. Fifty-three patients were enrolled at 21 institutions in Japan.
In 66.0 % (n = 35), partial remissions were achieved. Thirty of these patients (56.6 %) maintained their 20 mg dose over time, whereas the schedule was reduced to 20 mg every other day in five (9.4 %) patients. Within the group that achieved stable disease (n = 14, 26.4 %), dose escalations to 30 mg and 40 mg were performed in 4 (7.5 %) and 2 (3.8 %) patients, respectively. Eight patients continued the 20 mg dose. Disease progression occurred in 3 cases (5.7 %), and one patient was not evaluable. Overall, afatinib 20 mg gave rise to a disease control rate of 92.5 %. Median PFS and time to treatment failure were 12.6 months and 9.7 months, respectively. Median OS had not been reached yet at the time of the analysis.
Grade ≥ 3 AEs occurred in 12 patients (22.6 %), including diarrhea in 4 patients (7.5 %). This was lower than the rates for grade ≥ 3 AEs and diarrhea observed in the phase III setting with afatinib 40 mg (49 % and 14.4 %, respectively) [11]. Afatinib plasma concentrations 9 days after the start of treatment showed no correlation with ORR or time to treatment failure, performance status, smoking, clinical staging, or AEs including diarrhea. The only significant correlation was noted for the EGFR mutation status, with higher plasma concentrations in the exon 19 deletion group compared to the L858R mutation cohort (p = 0.03). Clinical activity was achieved even at low plasma concentrations with an average of 11.4 ng/ml. The authors pointed out that afatinib 20 mg might be considered a standard therapy for patients with EGFR-mutated NSCLC based on these findings.
European use of EGFR TKI therapy
The retrospective multinational study REFLECT assessed treatment and testing patterns as well as outcomes and attrition rates in the context of first-line EGFR TKI therapy with first- and second-generation agents in Austria, Bulgaria, Greece, Israel, Poland, Romania, Slovenia and Switzerland [12]. Overall, 896 patients with locally advanced or metastatic EGFR-mutant NSCLC who started treatment with afatinib, gefitinib or erlotinib between January 1, 2015, and June 30, 2018, were included in the analysis. REFLECT is one of the largest attrition rate studies investigating first-line treatment with first- or second-generation EGFR TKIs that has been conducted in European patients.
Afatinib, erlotinib and gefitinib were administered in 45.4 %, 27.3 % and 27.2 %, respectively. At the time of data collection, 85.4 % of patients had discontinued treatment. Median time to discontinuation amounted to 12.6 months in the first line. Radiographic progression was the main reason for discontinuation across the treatment lines, with increasing proportions of patients not receiving any next-line treatment (Figure 3). Among patients progressing on first-line EGFR TKIs (n=723), only 513 (71.0 %) were tested for the presence of the T790M resistance mutation. In these, the mutation was found in 58.3 %. Osimertinib was prescribed for 94.6 % of patients with confirmed T790M mutations in any subsequent line, mostly in the second line. Additionally, osimertinib in any subsequent line was used by 41 patients (18.5 %) with negative T790M test results and by 15 (6.2 %) who had not undergone T790M testing.
Median PFS and OS from the start of the first-line EGFR TKI therapy were 13.0 and 26.2 months. As the authors noted in their summary, this study indicates suboptimal survival in patients with EGFR-mutant NSCLC treated with first- and second-generation EGFR TKIs. To improve outcomes, a better understanding of the efficient use of EGFR TKIs is highly needed. However, the treatment landscape is anticipated to change after first-line approval of the third-generation EGFR TKI osimertinib, and further real-world evidence is awaited.
Figure 3: Attrition rates in the REFLECT study and reasons for treatment discontinuation across four treatment lines
REFERENCES
- Haura EB et al., JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). J Clin Oncol 37, 2019 (suppl; abstr 9009)
- Ahn MJ et al., Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1-2 study. Lancet Oncol 2020; 20(21): 1681-1690
- Kim SW et al., Intracranial anti-tumor activity of lazertinib in patients with advanced NSCLC who progressed after prior EGFR TKI therapy: Data from a phase I/II study. J Clin Oncol 38: 2020 (suppl; abstr 9571)
- Cho BC et al., Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in combination with lazertinib, a third-generation EGFR tyrosine kinase inhibitor, in advanced EGFR-mutant NSCLC. ESMO 2020, 1258O
- Larsen AK et al., Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther 2011; 131(1): 80-90
- Zhang L et al., ACTIVE: apatinib plus gefitinib versus placebo plus gefitinib as first-line treatment for advanced epidermal growth factor receptor-mutant non-small-cell lung cancer: a multicenter, randomized, double-blind, placebo-controlled phase III trial (CTONG1706). ESMO 2020, LBA50
- Toi Y et al., A randomized phase II study of osimertinib with or without bevacizumab in advanced lung adenocarcinoma patients with EGFR T790M mutation. ESMO 2020, 1259O
- Tan CS et al., Third generation EGFR TKIs: current data and future directions. Mol Cancer 2018; 17(1): 29
- Yu HA et al., Efficacy and safety of patritumab deruxtecan (U3-1402), a novel HER2 directed antibody drug conjugate, in patients with EGFR-mutated NSCLC. ESMO 2020, LBA62
- Noro R et al., A prospective, phase II trial of low-dose afatinib monotherapy for patients with EGFR mutation-positive non-small cell lung cancer (TORG1632). ESMO 2020, 1365P
- Kato T et al., Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: Subgroup analysis of LUX-Lung 3. Cancer Sci 2015; 106(9): 1202-1211
- Addeo A et al., Real-world treatment patterns, clinical outcomes and EGFR/T790M testing practices in patients with EGFR-mutated advanced NSCLC and 1L EGFR TKI therapy – a retrospective multinational study (REFLECT). ESMO 2020, 1299P
© 2020 Springer-Verlag GmbH, Impressum
More posts
Interview – Malignant mesothelioma: implementation of immunotherapy-based standards
Which outcomes can be expected in European patients with malignant mesothelioma who receive the current standard treatment?
EGFR-targeted options in a changing treatment landscape
The combination of amivantamab, a bispecific antibody that targets both EGFR and MET, and the potent third-generation EGFR TKI lazertinib is being explored in patients with advanced NSCLC. Amivantamab has demonstrated clinical activity across various types of EGFR-mutant NSCLC harboring both activating and resistance mutations and was granted FDA Breakthrough Therapy Designation for EGFR-mutant NSCLC with exon 20 insertion after progression on platinum-based chemotherapy.
Innovative and established approaches for patients with uncommon mutations
The highly potent, brain-penetrant, third-generation ALK tyrosine kinase inhibitor lorlatinib has been widely approved for the treatment of patients with ALK-positive advanced NSCLC who have previously received ALK TKIs. In the first-line setting, lorlatinib was compared with crizotinib in the randomized, phase III CROWN study that included almost 300 patients with stage IIIB/IV, ALK-positive NSCLC. Solomon et al.
Interview – Exploring interactions between radiotherapy and the immune system
The modulation of molecular pathways that determine the patient response to radiotherapy might contribute to improving patient outcomes. What insights have been gained to date in this field of research that may be relevant in the years to come?
Determinants of treatment success in small-cell lung cancer
The randomized, controlled, open-label, phase III CASPIAN trial has assessed first-line treatment with durvalumab ± tremelimumab plus platinum/etoposide (EP) compared to EP alone in patients with extensive-stage small-cell lung cancer (ES-SCLC). Durvalumab plus EP significantly improved OS compared to EP alone (HR, 0.73; p = 0.0047).
Early-stage lung cancer: noteworthy findings for different types of therapy
Postoperative radiotherapy (PORT) in patients with completely resected NSCLC has been a subject of debate for years. In the absence of robust data confirming the benefit of this intervention, its feasibility was additionally challenged by a multitude of changes that have taken place over the last two decades with respect to patient selection, (neo)adjuvant chemotherapy, surgery, and radiotherapy.