Air-pollution–induced lung cancer: defining a targetable link
The close association between air pollution and increased lung cancer risk has been known for decades, although causation remained unknown. Lung cancer in never smokers is characterized by a low mutational burden and the absence of a carcinogen-induced DNA mutation signature. In general, evidence against the classical mutation model explaining tumor growth as a result of DNA mutations has emerged, thus raising the need for an alternative model. The model initially developed by Berenblum in 1947 states that for environmental carcinogens to cause cancer, promoters need to act on pre-existing potential driver mutations (i.e., initiators), thus leading to clonal outgrowth [1, 2].
The role of PM2.5
Atmospheric particulate matter with a diameter ≤ 2.5 μm (PM2.5) represents the smallest particles of air pollution and is a major risk factor for various diseases [3, 4]. In terms of global mortality contribution, PM2.5 equals tobacco. More than 99 % of the world’s population live in areas where pollution exceeds the WHO-recommended PM2.5 threshold [5]. Five times more people are exposed to PM2.5 than to tobacco, although the relative risk is 15- to 20-fold lower than for smoking.
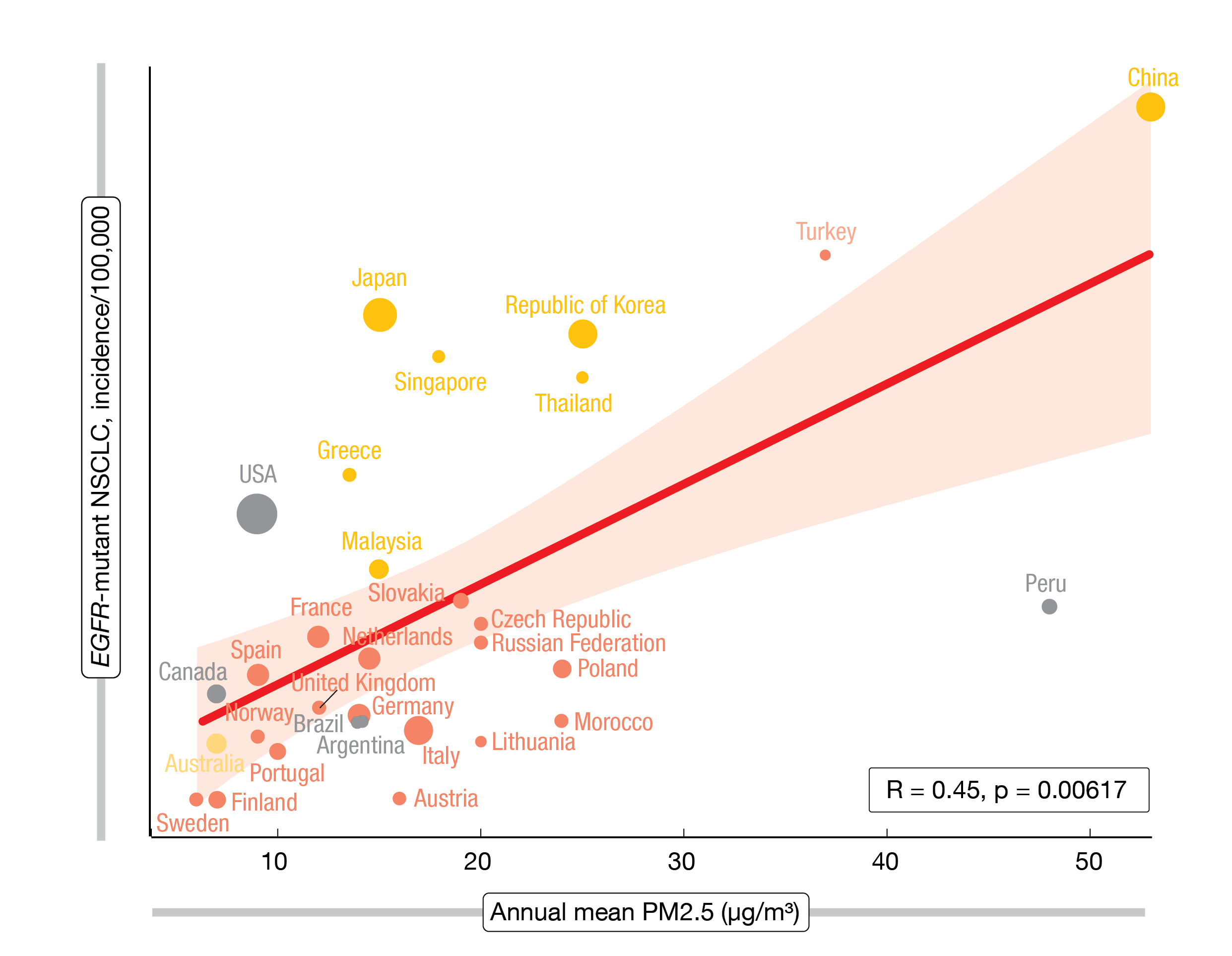
Swanton et al. found that the geographic distribution of EGFR-mutant lung cancer can be linked to PM2.5 concentrations, with a significant association between PM2.5 exposure and lung cancer incidence across the globe (Figure) [6]. This is an important observation as EGFR-mutant NSCLC occurs 4 to 5 times more frequently in never smokers than in smokers [7]. Based on three mouse models with pre-existing EGFR and KRAS mutations, the researchers demonstrated that air pollution indeed promotes cancer. Dose-dependent increases in the numbers of tumors and adenoma-to-carcinoma transitions were observed independent of the underlying driver oncogene.
Figure: Association between the incidence of EGFR-mutant NSCLC and the annual mean PM2.5 concentrations (international data)
Promotion of a cancer-stem-cell–like state
With respect to the direct link between pollution and cancer, the researchers assessed the essential role of the alveolar type 2 (AT2) progenitor cell, which is the most common cell of origin for EGFR-mutant lung cancer. Inflammation, specifically IL-1β, promotes alveolar regeneration by mobilizing AT2 cells. IL-1β can induce a primed AT2 progenitor state [8]. In the mouse model, exposure to pollution gave rise to an AT2 progenitor state transcriptional signature, as well as increased influx of macrophages that release IL-1β. While neither air pollution nor the EGFR mutation alone appeared to be sufficient to augment a stem-cell state, stem cell capacity was shown to require both, with the EGFR mutation representing the initiator and pollution representing the promoter.
The role of inflammation for the pollution-induced tumor progenitor function was explored in the COPA study (NCT02236039) that included healthy never smokers who received either PM2.5 or filtered air for 2 hours, with the PM2.5 exposure levels being equivalent to those frequently encountered in major Asian cities. Bronchial brushing was taken 24 hours after exposure, and a transcriptomic analysis was performed via RNA sequencing. The results were compared with those obtained in mice.
According to this, PM2.5 drove IL-1β release from lung epithelium and alveolar macrophages in both species. IL-1β actually mimicked PM2.5 in a stem cell progenitor assay. This fits with data from the CANTOS trial showing that the anti-IL-1β antibody canakinumab reduced lung cancer incidence and mortality [9]. In EGFR-mutant mice exposed to pollution, simultaneous anti-IL-1β antibody treatment completely abrogated the growth of tumors.
Mutations even in healthy tissue
An important prerequisite for the tumor promotion model developed by Berenblum is the presence of pre-existing mutations in latent cells. Indeed, studies of normal lung samples demonstrated that activating EGFR and KRAS mutations were present in 15 % and 53 %, respectively. These lung samples were obtained from never smokers, and it was found that cancer driver mutations increase with age.
Taken together, the results explain the absence of a mutagenic signature associated with pollution. IL-1β releases appear to act on mutant clones in histologically normal tissue harboring oncogenic mutations, leading to trans-differentiation to a progenitor stem-cell state that ultimately induces tumor formation. Thus, air pollution may promote cancer without directly causing DNA mutations.
These findings might facilitate molecular cancer prevention in high-risk populations. Moreover, other environmental carcinogens that do not mutate DNA might operate through similar, potentially actionable inflammatory pathways. Also, the question of whether UV light and tobacco act as both initiator and promoter raises concerns. These results provide a mandate to lower PM2.5 concentrations with the aim to improve long-term health outcomes in pollution-exposed populations.
REFERENCES
- Berenblum I, Shubik P, A new, quantitative, approach to the study of the stages of chemical carcinogenesis in the mouse’s skin. Br J Cancer 1947; 1(4): 383-391
- Balmain A, The critical roles of somatic mutations and environmental tumor-promoting agents in cancer risk. Nat Genet 2020; 52(11): 1139-1143
- Katsouyanni K et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology 2001; 12: 521-531
- Klemm RJ et al., The impact of frequency and duration of air quality monitoring: Atlanta, GA, data modeling of air pollution and mortality. J Air Waste Manag Assoc 2011; 61: 1281-1291
- https://www.who.int/news/item/04-04-2022-billions-of-people-still-breathe-unhealthy-air-new-who-data
- Swanton C et al., Mechanism of action and an actionable inflammatory axis for air pollution induced non-small cell lung cancer: Towards molecular cancer prevention. ESMO 2022, abstract LBA1
- Mazières J et al., Specificities of lung adenocarcinoma in women who have never smoked. J Thorac Oncol 2013; 8(7): 923-929
- Choi J et al., Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell 2020; 27(3): 366-382.e7
- Ridker PM et al., Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390(10105): 1833-1842
© 2022 Springer-Verlag GmbH, Impressum
More posts
New therapeutic options being currently investigated in advanced or metastatic colorectal cancer
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, and it is the fourth most frequent cancer diagnosis.A current treatment option for RAS and BRAF wild-type (WT) metastatic colorectal cancer (mCRC) is the chemotherapy doublet (FOLFOX/FOLFIRI) with an anti-EGFR monoclonal antibody (cetuximab or panitumumab).
An update and future directions in advanced gastric or gastrointestinal junction cancer (G/GEJC)
With more than 1 million newly diagnosed cases in 2020, gastric cancer (GC) is the fifth most frequent cancer; it was also the third leading cause of cancer-related death worldwide. Gastroesophageal junction (GEJ) cancer concerns a form of gastric cancer developing around the digestive tract where esophagus and stomach connect; in the last years, the prevalence of GEJ constantly increased.
Innovative combinations in esophageal squamous cell carcinoma
Each year, esophageal cancer (EC) is responsible for more than half a million deaths worldwide. Among them, esophageal squamous cell carcinoma (ESCC) accounts for the vast majority (~ 85 %) of EC incidences . At diagnosis, 70 % of ESCC is unresectable [3] and the 5-year survival rate is limited (30 % - 40 %). Patients with advanced or metastatic ESCC have a poor prognosis; their overall survival (OS) after standard first-line chemotherapy is limited to less than a year and other treatment options are scarce.
Novel agents or combinations in recurrent or metastatic nasopharyngeal cancer
Nasopharyngeal cancer (NPC) is a rare malignancy with an incidence of approximately 133,000 annually worldwide, resulting in about 80,000 deaths per year. Whereas early-stage and locally advanced NPC have a good prognosis, treatment of recurrent or metastatic nasopharyngeal cancer is a challenging; it is thus associated with a poor prognosis, especially in patients who have failed two or more lines of systemic therapy, with a median progression-free survival (mPFS) of seven months and median overall survival (mOS) of 22 months.
Preface ASCO Solid Tumor 2022
After 2 years of the COVID-19 pandemic, the Annual Meeting of the American Society of Clinical Oncology (ASCO), was held in Chicago, USA, and virtually from 3rd–7th June 2022.As always, the very much-anticipated event brought leading experts from across the globe together to learn and discuss the groundbreaking updates and scientific advancements which were covered in more than 2,000 abstracts, along with 85 livestream sessions, and more than 2,500 poster presentations.