Innovative treatment approaches: non–EGFR-directed bispecific antibodies and ADCs
HARMONi-2: ivonescimab
In the management of patients with advanced PD-L1–positive non–small-cell lung cancer (NSCLC), the use of bispecific antibodies is gaining ground. Ivonescimab (AK112) is a first-in-class bispecific antibody directed against PD-1 and the vascular endothelial growth factor (VEGF) type A. As VEGF and PD-(L)1 inhibitors were found to have synergistic activity, it was postulated that their simultaneous use might enhance treatment efficacy compared with the co-administration of separate anti–PD-(L)1 and anti-VEGF agents [1-3]. Promising clinical efficacy and safety have already been demonstrated with first-line ivonescimab in the phase II setting [4]. The randomized, double-blind, phase III HARMONi-2 study was designed to compare ivonescimab 20 mg/kg Q3W with pembrolizumab 200 mg Q3W in untreated patients with PD-L1–positive (TPS ≥ 1 %), stage IIIB-IV NSCLC. Overall, the study population comprised 398 patients, with approximately half randomized into each arm. Progression-free survival (PFS) by blinded independent review constitutes the primary endpoint.
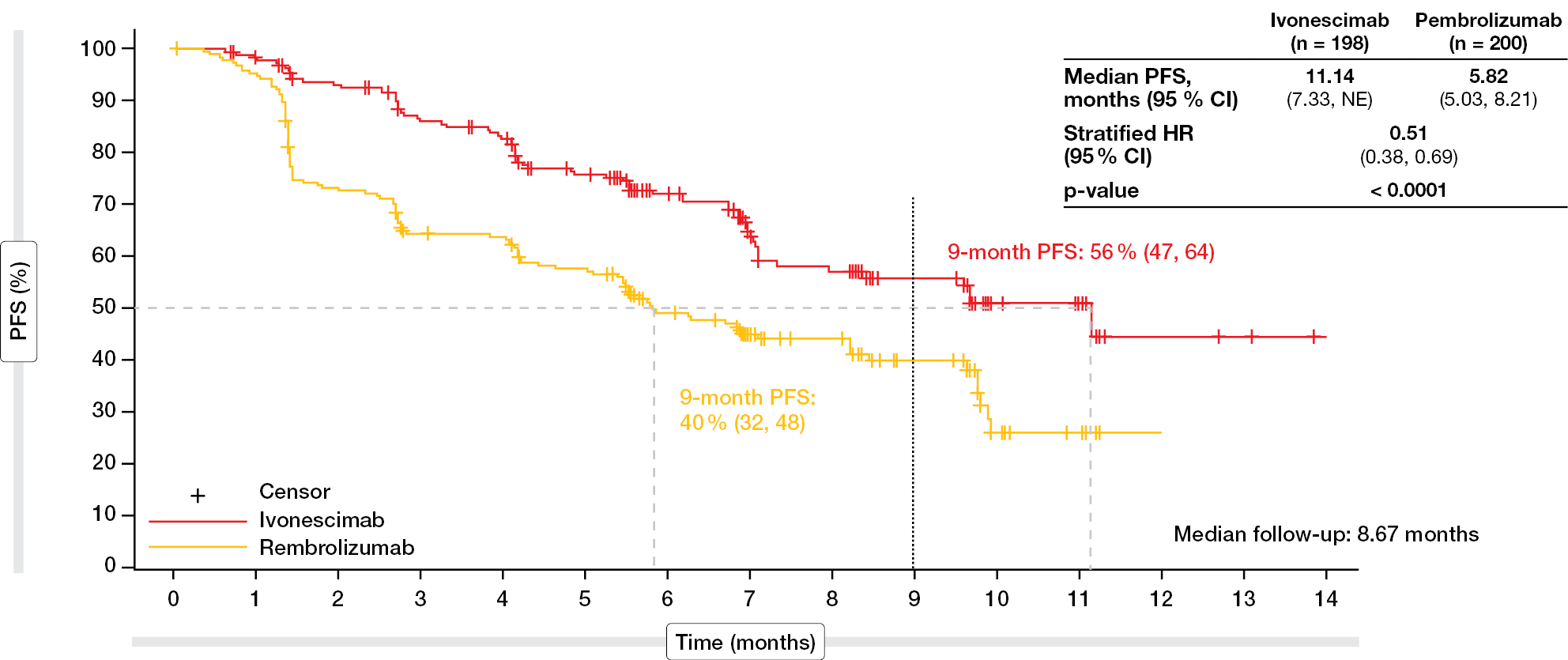
According to the results of the interim analysis reported by Zhou et al. at WCLC 2024 after a median follow-up of 8.67 months, PFS was significantly improved by 5.3 months with ivonescimab vs. pembrolizumab (median, 11.14 vs. 5.82 months; p < 0.0001; Figure 1) [5]. This difference translated into a 49 % risk reduction (HR, 0.51). At 9 months after initiation of treatment, 56 % vs. 40 % of patients were alive and progression-free. The PFS advantage was consistent across major clinical subgroups. Notably, ivonescimab-treated patients fared better irrespective of PD-L1 expression (HRs, 0.54 and 0.46 for the cohorts with TPS 1-49 % and ≥ 50 %, respectively) and histology (HRs, 0.48 and 0.54 for squamous and non-squamous histology, respectively). All of these subgroups experienced meaningful PFS improvement with ivonescimab vs. pembrolizumab. Similarly, the overall response rate (ORR) was higher in the experimental arm than in the control arm (50.0 % vs. 38.5 %), and a greater proportion of patients achieved disease control (89.9 % vs. 70.5 %).
Figure 1: Significant improvement of progression-free survival with ivonescimab vs. pembrolizumab in PD-L1–positive advanced NSCLC
Low rates of burdensome AEs
The safety profile of ivonescimab was consistent with prior studies, and the treatment exhibited high tolerability. Among treatment-related adverse events (TRAEs), proteinuria ranked first (all grades, 31.5 %; grade ≥ 3, 3.0 %), followed by AST increases (19.8 %), hypercholesterolemia (16.2 %), increases in blood bilirubin levels (15.7 %) and hypertension (15.7 %). Serious TRAEs emerged in 20.8 % vs. 16.1 %, and in 1.5 vs. 3.0 %, TRAEs led to treatment discontinuation. Fatal TRAEs were reported in 0.5 % vs. 1.0 %. The researchers performed a separate safety analysis of the subgroup with squamous histology and found that ivonescimab proved tolerable in this cohort as well. Here, serious TRAEs were seen in 19 % in both arms, and discontinuation was necessary in 2.2 % vs. 3.3 % with ivonescimab vs. pembrolizumab.
Immune-related AEs (irAEs) reported for ivonescimab were similar to those observed for the PD-1 inhibitor. Grade ≥ 3 irAEs occurred in 7.1 % vs. 8.0 % and serious irAEs in 5.6 % vs. 11.1 %. Treatment discontinuation due to irAEs was noted in 0 % vs. 2.5 %. None of the irAEs led to death in either arm. Possible VEGF-related AEs emerged in 47.7 % vs. 21.1 %, with grade ≥ 3 events observed in 10.2 % vs. 1.0 %. All VEGF-related AEs were grade 1-3 in both arms. Two patients with non-squamous histology developed grade 3 hemorrhage. Assessment of the global health status according to the EORTC QLQ-C30 questionnaire revealed numerical improvement of the time to deterioration in the experimental arm compared to the control arm. The overall survival (OS) results were not mature at the time of the analysis and will be reported in the future.
As the authors emphasized in their conclusion, HARMONi-2 is the first randomized phase III study to demonstrate clinically significant improvement in efficacy with a novel drug compared to pembrolizumab in patients with advanced NSCLC. Considering these findings, ivonescimab is a new first-line treatment option in the setting of advanced, PD-L1–expressing (TPS ≥ 1 %) NSCLC.
Final OS data from TROPION-Lung01
The randomized, open-label, global phase III TROPION-Lung01 study was initiated to assess the TROP-2–directed antibody drug conjugate (ADC) datopotamab deruxtecan (Dato-DXd) 6 mg/kg Q3W (n = 299) in pretreated patients with stage IIIB, IIIC or IV NSCLC with or without actionable genomic alterations. Dato-DXd was compared with docetaxel 75 mg/m2 Q3W (n = 305). The group without genomic alterations had already received one or two prior lines including platinum-based chemotherapy and anti-PD-(L)1 antibody therapy; in the cohort with alterations (i.e., EGFR, ALK, NTRK, BRAF, ROS1, MET exon 14 skipping, RET), patients were pretreated with one or two approved targeted therapies plus platinum-based chemotherapy and ≤ 1 anti–PD-(L)1 antibody. TROPION-Lung01 met its dual primary endpoint of PFS, with Dato-DXd giving rise to a significant improvement compared to docetaxel (4.4 vs. 3.7 months; HR, 0.75; p = 0.004) [6]. The PFS benefit was mainly driven by the subgroup with non-squamous histology that derived a 37 % relative risk reduction (5.6 vs. 3.7 months; HR, 0.63).
At WCLC 2024, the final analysis of the dual endpoint of OS showed a numerical improvement in the Dato-DXd arm that did not reach statistical significance (12.9 vs. 11.8 months; HR, 0.94; p = 0.530) [7]. The 2-year OS rates were 25.8 % vs. 20.2 %. In patients with non-squamous histology, median OS was 14.6 vs. 12.3 months (HR, 0.84), with 2-year OS rates of 29.0 % vs. 21.7 %. Dato-DXd showed consistent benefits across all efficacy endpoints in the non-squamous subgroup: OS improvements were observed regardless of the actionable genomic alteration status and subsequent use of docetaxel after failure of therapy. Even the effect of all post-treatment anticancer therapies in both arms did not impact the OS benefit according to a sensitivity analysis.
In the cohort with squamous histology, on the other hand, Dato-DXd–treated patients fared worse with regard to OS than those receiving docetaxel (7.6 vs. 9.4 months; HR, 1.32).
The safety analysis demonstrated no late-onset toxicities at an additional follow-up of approximately 11 months after the prior PFS data cutoff. No new adjudicated drug-related interstitial lung disease (ILD) events or deaths had occurred. Comparatively fewer grade ≥ 3 TRAEs were observed in the experimental arm (26 % vs. 42 %), and fewer TRAEs caused dose reductions (20 % vs. 30 %) or discontinuations (8 % vs. 12 %). Median treatment durations for Dato-DXd and docetaxel were 4.2 and 2.8 months, respectively. The full safety analysis set and the non-squamous subgroup showed similar safety profiles. In their entirety, these results support the use of Dato-DXd as a new therapeutic option for patients with previously treated non-squamous NSCLC eligible for subsequent therapy.
TROP2 QCS-NMR as predictive biomarker for Dato-DXd
It has been shown that conventional immunohistochemistry (IHC) scoring does not predict responses to TROP2-directed ADCs in patients with NSCLC [8, 9]. Based on the hypothesis that a more precise and quantitative assessment of TROP2 expression on the cell membrane and in the cytoplasm might predict efficacy of Dato-DXd, Garassino et al. evaluated the TROP2 normalized membrane ratio (NMR) as measured by quantitative continuous scoring (QCS) [10]. This marker reflects the expression of TROP2 in the membrane relative to total TROP2 (i.e., membrane and cytoplasm). QCS is a fully supervised computational pathology approach that precisely quantifies and locates targets such as TROP2.
This exploratory analysis of TROPION-Lung01 was based on the biomarker-evaluable population (n = 352), which included patients with non-squamous histology with and without actionable genomic alterations (NSQ/AGA and NSQ/non-AGA, respectively), as well as a group with squamous histology (SQ). TROP2 QCS-NMR positivity was defined as a TROP2 NMR ≤ 0.56 in < 75 % of tumor cells, while patients with a TROP2 NMR ≤ 0.56 in ≥ 75 % of tumor cells were considered negative. The cut-points were confirmed through a robust statistical analysis plan (including bootstrapping, cross validation, and sensitivity analyses) and replication.
TROP2 QCS-NMR positivity was more prevalent in NSQ patients than in SQ patients (66 % and 44 %, respectively). Within the NSQ group, the AGA cohort showed a higher positivity rate than the non-AGA cohort (76 % and 63 %, respectively). The assessment of PFS according to TROP2 QCS-NMR status demonstrated that TROP2 QCS-NMR positivity was predictive for longer PFS with Dato-DXd in the biomarker-evaluable population (Figure 2). The same finding emerged for the NSQ/non-AGA biomarker-evaluable group. With regard to safety, the analysis revealed similar overall AE rates and grade ≥ 3 rates with Dato-DXd regardless of the TROP2 QCS-NMR status. In their summary, the scientists noted that TROP2 QCS-NMR has the potential to be the first TROP2 biomarker and the first computational pathology biomarker for predicting clinical response to Dato-DXd in the setting of NSCLC. Further investigation of TROP2 QCS-NMR in patients with advanced NSCLC is ongoing in the first-line trials AVANZAR (NCT05687266) and TROPION-Lung 10 (NCT06357533).
Figure 2: TROPION-Lung01: efficacy of Dato-DXd by TROP2 QCS-NMR status
Telisotuzumab vedotin: LUMINOSITY
Telisotuzumab vedotin (Teliso-V) is the first ADC to target c-Met–overexpressing tumor cells that are found in approximately 25 % of patients with non-squamous, EGFR-wildtype NSCLC [11]. Further research showed that c-Met overexpression correlates with decreased OS in this group, including patients of Asian origin [12, 13]. Teliso-V was evaluated in the non-randomized phase II LUMINOSITY trial with the aim of identifying the optimal population with c-Met protein-overexpressing advanced NSCLC. Indeed, while the squamous and EGFR-mutant non-squamous cohorts met the stopping criteria, the EGFR-wildtype non-squamous cohort met the criteria for expansion in stage 2 of the study. Horinouchi et al. presented the results for patients of Asian ethnicity with c-MET–overexpressing, EGFR-wildtype NSCLC of non-squamous histology [14]. This group had previously received ≤ 2 prior lines of systemic therapy in the advanced setting, including cytotoxic chemotherapy (≤ 1 line), immunotherapy (sequential or combined with chemotherapy), and targeted therapy in the presence of appropriate driver aberrations. Among 57 patients with Asian ethnicity, 48 belonged to the efficacy-evaluable cohort. Approximately half each showed high and intermediate c-Met expression. Their median number of prior systemic anticancer therapies was 1, with platinum-based and immune checkpoint inhibitor-based treatment having been administered in 92 % and 77 %, respectively.
Treatment with Teliso-V induced compelling and durable responses per independent review, especially in patients with high c-Met expression. The ORR and DCR in the total group were 35.4 % and 66.7 %, respectively (Table 1). Patients with high c-MET expression (n = 26) showed an ORR of 46.2 %, while this was 22.7 % in those with intermediate c-MET (n = 22). Median duration of response (DOR) ≥ 6 months resulted in 50.0 % and 40.0 %, respectively. As the authors pointed out, these findings were comparable with those observed in the overall study population that included various ethnicities. Teliso-V gave rise to median PFS and OS of 5.5 and 17.4 months, respectively. OS was longer in the c-Met–high group than in the c-Met–intermediate group (25.4 vs. 17.0 months).
Teliso-V demonstrated an acceptable and clinically manageable safety profile. Late-onset peripheral sensory neuropathy constituted the most common TRAE (any grade, 21.1 %; grade ≥ 3, 1.8 %). Further TRAEs included hypoalbuminemia (19.3 %), ALT increases (17.5 %) and blurred vision (15.8 %). Retrospectively adjudicated drug-related ILD occurred in 15.8 %, with 8.8 % of patients developing grade ≥ 3 events and two (3.5 %) dying, although one of these two adjudicated deaths was attributed to disease progression. The global, randomized phase III TeliMET NSCLC-01 study is currently comparing Teliso-V to docetaxel in pretreated patients with advanced, c-Met–overexpressing, non-squamous, EGFR-wildtype NSCLC (NCT04928846).
Tusamitamab ravtansine in NSCLC with CEACAM5 expression
Another target is the transmembrane glycoprotein CEACAM5, which is highly expressed in up to 25 % of non-squamous NSCLC cases [15]. Efficacy and safety of the CEACAM5-targeting ADC tusamitamab ravtansine (Tusa-rav) were explored in the phase III CARMEN-LC03 trial that included patients with advanced non-squamous NSCLC after platinum-based chemotherapy and immune checkpoint inhibitor treatment. Their tumors showed CEACAM5 expression, i.e. ≥ 2+ in ≥ 50 % of tumor cells according to IHC. Tusa-rav 100 mg/m2 Q2W (n = 194) was compared with docetaxel 75 mg/m2 Q3W (n = 195). PFS per independent review and OS were defined as the dual primary endpoints. Besse et al. presented the final PFS and interim OS results at WCLC 2024 [16].
The CARMEN-LC03 trial did not meet its dual primary endpoints and was terminated early. Median PFS was 5.4 vs. 5.9 months for Tusa-rav vs. docetaxel (HR, 1.14; p = 0.8204). For OS, there was a positive trend (12.8 vs. 11.5 months; HR, 0.85; p = 0.112). In addition, subgroup analyses suggested trends favoring Tusa-rav in patients with CEACAMS5 expression ≥ 80 % regarding PFS (HR, 0.866) and OS (HR, 0.711). The assessment of health-related quality of life again yielded trends with respect to time to deterioration; this applied to disease-related symptoms (2.8 vs. 1.9 months) as well as physical functioning (7.5 vs. 4.2 months) and role functioning (5.6 vs. 4.2 months). Moreover, Tusa-rav induced fewer grade ≥ 3 TRAEs (14.9 % vs. 39.5 %), serious AEs (6.2 % vs. 20.3 %) and TRAEs leading to definitive treatment discontinuation (7.7 % vs. 16.9 %). At 25.8 %, ocular events (i.e., keratopathy, keratitis) were the most common TRAEs in the experimental arm; these rates were consistent with those reported previously.
The authors noted that a potential limitation of the study was the use of archived rather than fresh tissue to evaluate the CEACAM5 expression status in most study participants. Also, it remains unclear how prior immunotherapy and/or chemotherapy might have affected the CEACAM5 expression before study inclusion. Moreover, an exploratory analysis aiming to understand the high performance of the control arm revealed a potential positive prognostic role of the CEACAM5 expression [17]. The CARMEN-LC03 trial was discontinued in December 2023, although patients benefiting from the treatment could continue to receive Tusa-rav.
MDM2-p53 antagonist brigimadlin
Therapeutic targeting is also possible at the level of p53 and its negative regulator MDM2 [18]. Amplification of the MDM2 gene (MDM2-amp) has been described in 4-6 % of patients with adenocarcinoma of the lung and is associated with poor prognosis [19-22]. Typically, co-occurring mutations are found alongside MDM2-amp; these include EGFR (40 %), KRAS (25 %), the METexon14 splice variant (13 %), and ALK rearrangement (7 %) [19]. The potent oral MDM2-p53 antagonist brigimadlin inhibits the interaction between p53 and MDM2, thus preventing MDM2 from inactivating p53 and restoring the function of p53 [23]. At WCLC 2024, Yamamoto et al. reported data on 24 pretreated patients with MDM2-amplified adenocarcinoma of the lung who participated in three Ia-IIb trials testing brigimadlin in advanced solid tumors [24]. Overall, 15 patients received brigimadlin as monotherapy at a dose of 45 mg Q3W, while nine were treated with brigimadlin 45 mg in addition to the PD-1 inhibitor ezabenlimab 240 mg Q3W.
Next generation sequencing in patients with available samples (n = 12) showed a rather complex genomic profile. Six patients had EGFR mutations, with three of them also showing EGFR amplification. In six patients, CDK4 co-amplification was found. Brigimadlin treatment gave rise to encouraging preliminary efficacy. Among 12 evaluable patients receiving single-agent brigimadlin, four achieved partial response (PR), two unconfirmed PR, and five stable disease. The combination (n = 8 evaluable patients) induced PR in one patient, unconfirmed PR in another, and stable disease in five. Responses were not correlated with the MDM2 copy numbers (Figure 3) and appeared durable; at the time of the analysis, 13 patients were still ongoing, which is the reason why duration of response could not be calculated. Overall, the duration of treatment with brigimadlin exceeded the time on previous treatment lines for many patients, as the authors noted.
The safety results were consistent with those observed in the overall study population. Serious AEs occurred in 27 %. The most common grade ≥ 3 AEs of special interest were neutropenia and decreased platelet count. Combination treatment with brigimadlin plus ezabenlimab did not induce ILD/pneumonitis in any patient. None of the AEs prompted treatment discontinuation. The scientists concluded that this analysis supports the continued investigation of brigimadlin in patients with adenocarcinoma of the lung.
Figure 3: No correlation between responses to brigimadlin and MDM2 copy numbers in three studies
SCLC: ifinatamab deruxtecan
In the setting of extensive-stage small-cell lung cancer (ES-SCLC), the B7-H3 (CD276)-directed ADC ifinatamab deruxtecan (I-DXd) is being evaluated at two doses in the phase II IDeate-Lung01 study. Prior to inclusion, patients have received ≤ 3 lines of systemic therapy including ≥ 1 line of platinum-based chemotherapy and have developed radiologically documented disease progression on or after the most recent systemic treatment. Enrollment of patients with asymptomatic brain metastases (either treated or untreated) was permitted. Overall, 88 individuals were randomized to either I-DXd 8 mg/kg Q3W (n = 46) or I-DXd 12 mg/kg Q3W (n = 42). The ORR by independent review constitutes the primary endpoint. Rudin et al. reported an interim analysis of IDeate-Lung01 at WCLC 2024 [25].
I-DXd demonstrated promising antitumor activity in this pretreated ES-SCLC setting. At both doses, the ADC induced rapid responses, although the 12 mg/kg dose, as compared to the 8 mg/kg dose, performed better with respect to ORR (54.8 % vs. 26.1 %) and DCR (90.5 % vs. 80.4 %). PFS was similar between the study arms, numerically favoring the 12 mg/kg dose (5.5 vs. 4.2 months). This was also true for OS (11.8 vs. 9.4 months). A subset of 16 patients with brain target lesions at baseline showed evidence of systemic and intracranial responses, with ORRs ranging from 16.7 % to 66.7 % (Table 2).
Median time on treatment was longer with the 12 mg/kg dose than with the 8 mg/kg dose (4.7 vs. 3.5 months). I-DXd displayed a generally manageable safety profile, although treatment-emergent AEs (TEAEs) led more frequently to drug discontinuation in the 12 mg/kg cohort than in the 8 mg/kg cohort (16.7 % vs. 6.5 %). Treatment-related TEAEs predominantly included nausea, decreased appetite, anemia, and decreased neutrophil count/neutropenia. ILD/pneumonitis adjudicated as treatment-related was reported in 11.9 % and 8.7 % of patients in the 12 mg/kg and 8 mg/kg cohorts, respectively. Most of these were grade 1 and 2. I-DXd 12 mg/kg has been selected as the recommended phase III dose for further clinical development. The phase III IDeate-Lung02 trial is ongoing in patients with relapsed SCLC after only one prior line of therapy (NCT06203210).
Findings for obrixtamig in LCNEC-L
Large-cell neuroendocrine carcinoma of the lung (LCNEC-L) is a rare and highly aggressive type of lung cancer. As these tumors share similarities with SCLC, treatment strategies are extrapolated from the SCLC setting, although standards of care are lacking [26-28]. Approximately 70 % of LCNEC-L tumors express DLL3, thus providing a target for agents such as the novel DLL3/CD3 T-cell–engaging bispecific antibody obrixtamig (BI 764532). A first-in-human, dose-escalation trial is testing obrixtamig in patients with advanced, DLL3-positive SCLC, LCNEC-L, and extrapulmonary neuroendocrine carcinomas (epNEC). DLL3 positivity was established according to central review. The patients had failed available standard therapies (≥ 1 line of platinum-based chemotherapy) or were ineligible for them. A total of 168 individuals had been treated at the time of data cutoff. At WCLC 2024, Wermke et al. reported results for the LCNEC-L cohort that included 14 patients [29]. In this group, 43 % and 29 % had previously received 2 and ≥ 3 lines of therapy, respectively. Seventy-one percent had been treated with PD-(L)1 inhibitors. Brain and liver metastases were present in 50 % and 36 %, respectively.
Patients with LCNEC-L did not develop any dose-limiting toxicities, and the maximum tolerated dose was not reached. Among TRAEs, cytokine release syndrome (CRS) and dysgeusia were most common with rates of 36 % each. This was followed by asthenia (21 %), nausea (21 %), decreased lymphocyte count (14 %) and fatigue (14 %). None of the TRAEs was grade ≥ 3. CRS mainly occurred early on and was manageable with step-in dosing and supportive care. In addition to its acceptable safety profile, obrixtamig demonstrated promising efficacy. Responses in the overall group including SCLC, LCNEC-L and epNEC occurred at dose levels ≥ 90 µg/kg. In the LCNEC-L subgroup, 70 % of ten evaluable patients achieved partial responses with obrixtamig ≥ 90 µg/kg while 20 % had disease stabilization, which resulted in a DCR of 90 %. The fact that five patients remained on treatment at the time of the analysis suggests durable responses. Further dose optimization is ongoing.
REFERENCES
- Manegold C et al., The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol 2017; 12(2): 194-207
- Zhao Y et al., VEGF/VEGFR-targeted therapy and immunotherapy in non-small cell lung cancer: targeting the tumor microenvironment. Int J Biol Sci 2022; 18(9): 3845-3858
- Coward J et al., Safety and efficacy of AK112, an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors in a phase I dose escalation study. J Clin Oncol 39, 2021 (suppl 15; abstr 2515)
- Wang L et al., A Phase 1b study of ivonescimab, a programmed cell death protein-1 and vascular endothelial growth factor bispecific antibody, as first- or second-line therapy for advanced or metastatic immunotherapy-naive NSCLC. Thorac Oncol 2024; 19(3): 465-475
- Zhou C et al., Phase 3 study of ivonescimab (AK112) vs. pembrolizumab as first-line treatment for PD-L1-positive advanced NSCLC: HARMONi-2. WCLC 2024, abstract PL02.04
- Lisberg A et al., Datopotamab deruxtecan vs docetaxel in previously treated advanced/metastatic non-small cell lung cancer: Results of the randomized phase III study TROPION-Lung01. ESMO 2023, abstract LBA12
- Sands J et al., Datopotamab deruxtecan vs docetaxel in patients with non-small cell lung cancer: Final overall survival from TROPION-Lung01. WCLC 2024, abstract OA08.03
- Shimizu T et al., First-in-human, phase I dose-escalation and dose-expansion study of trophoblast cell-surface antigen 2-directed antibody-drug conjugate datopotamab deruxtecan in non-small-cell lung cancer: TROPION-PanTumor01. J Clin Oncol 2023; 41(29): 4678-4687
- Heist RS et al., Therapy of advanced non-small-cell lung cancer with an SN-38-anti-trop-2 drug conjugate, sacituzumab govitecan. J Clin Oncol 2017; 35(24): 2790-2797
- Garassino M et al., Normalized membrane ratio of TROP2 by quantitative continuous scoring is predictive of clinical outcomes in TROPION-Lung01. WCLC 2024, abstract PL02.11
- Motwani M et al., Prevalence of c-Met overexpression (c-Met+) and impact of prior lines of treatment on c-Met protein expression in NSCLC. J Thorac Oncol 2021; 16(10): S1169-S1170
- Liang H et al., MET oncogene in non-small cell lung cancer: Mechanism of MET dysregulation and agents targeting the HGF/c-Met axis. Onco Targets Ther 2020; 13: 2491-2510
- Huang L et al., MET expression plays differing roles in non-small-cell lung cancer patients with or without EGFR mutation. J Thorac Oncol 2014; 9(5): 725-728
- Horinouchi H et al., Telisotuzumab vedotin in Asian patients with c-Met protein-overexpressing non-squamous EGFR WT NSCLC: Results from LUMINOSITY. WCLC 2024, abstract OA16.03
- Lefebvre AM et al., The search for therapeutic targets in lung cancer: Preclinical and human studies of carcinoembryonic antigen-related cell adhesion molecule 5 expression and its associated molecular landscape. Lung Cancer 2023; 184: 107356
- Besse B et al., Tusamitamab ravtansine vs docetaxel in previously treated advanced nonsquamous NSCLC: Results from phase 3 CARMEN-LC03 trial. WCLC 2024, abstract OA08.05
- Cho BC et al., Exploratory analyses of tusamitamab ravtansine vs docetaxel in previously treated non-squamous NSCLC patients: CARMEN-LC03. WCLC 2024, abstract P2.10B.01
- Rudolph D et al., BI 907828: a novel, potent MDM2 inhibitor that is suitable for high-dose intermittent schedules. Cancer Res 2018; 78(Suppl 13): abstract 4868
- Elkrief A et al., Combination therapy with MDM2 and MEK inhibitors is effective in patient-derived models of lung adenocarcinoma with concurrent oncogenic drivers and MDM2 amplification. J Thorac Oncol 2023; 18(9): 1165-1183
- Dworakowska D et al., MDM2 gene amplification: a new independent factor of adverse prognosis in non-small cell lung cancer (NSCLC). Lung Cancer 2004; 43(3): 285-295
- Sun D et al., Targeting MDM2 in malignancies is a promising strategy for overcoming resistance to anticancer immunotherapy. J Biomed Sci 2024; 31: 17
- Sinha A et al., Early-stage lung adenocarcinoma MDM2 genomic amplification predicts clinical outcome and response to targeted therapy. Cancers (Basel) 2022; 14(3): 708
- Sun D et al., Primary resistance to first-generation EGFR-TKIs induced by MDM2 amplification in NSCLC. Mol Med 2020; 26: 66
- Yamamoto N et al., Efficacy and safety of brigimadlin, a MDM2-p53 antagonist, in patients with advanced lung adenocarcinoma. WCLC 2024, abstract P3.12C.03
- Rudin CM et al., Ifinatamab deruxtecan (I-DXd) in extensive-stage small cell lung cancer (ES-SCLC): Interim analysis of Ideate-Lung01. WCLC 2024, abstract OA04.03
- Andrini E et al., Large cell neuroendocrine carcinoma of the lung: Current understanding and challenges. J Clin Med 2022; 11(5): 1461
- Corbett V et al., Management of large cell neuroendocrine carcinoma. Front Oncol 2021; 11: 653162
- Lo Russo G et al., Treatment of lung large cell neuroendocrine carcinoma. Tumour Biol 2016; 37(6): 7047-7057
- Wermke M et al., Phase I trial of DLL3/CD3 IgG-like T-cell engager obrixtamig (BI 764532) in patients with DLL3-positive tumors: Patients with LCNEC-L. WCLC 2024, abstract OA10.05
© 2024 Springer-Verlag GmbH, Impressum
More posts
Innovative treatment approaches: non–EGFR-directed bispecific antibodies and ADCs
In the management of patients with advanced PD-L1–positive non–small-cell lung cancer (NSCLC), the use of bispecific antibodies is gaining ground. Ivonescimab (AK112) is a first-in-class bispecific antibody directed against PD-1 and the vascular endothelial growth factor (VEGF) type A. As VEGF and PD-(L)1 inhibitors were found to have synergistic activity, it was postulated that their simultaneous use might enhance treatment efficacy compared with the co-administration of separate anti–PD-(L)1 and anti-VEGF agents [1-3].
HER2 aberrations and other oncogenic drivers
HER2 mutations are rare as they occur in approximately 2–4 % of NSCLC cases, although they are associated with poor prognosis and increased incidence of brain metastases [1, 2]. As HER2-mutant NSCLC is relatively insensitive to chemotherapy, there is a substantial unmet need for targeted options [3, 4].
Concurrent MET/EGFR inhibition and third-generation EGFR TKIs
The EGFR-MET bispecific antibody amivantamab in addition to the third-generation EGFR tyrosine kinase inhibitor (TKI) lazertinib has shown favorable results as first-line treatment compared to osimertinib in the ongoing randomized, double-blind, phase III MARIPOSA trial. A total of 1,074 patients from 27 countries with advanced non–small-cell lung cancer (NSCLC) and activating EGFR mutations were enrolled in the study.
Preface – WCLC 2024
It is our pleasure to present highlights from the 2024 World Conference on Lung Cancer, held from September 7th to 10th in San Diego, USA. Our first chapter focuses on the evolving landscape of concurrent MET/EGFR inhibition and novel third-generation EGFR tyrosine kinase inhibitors (TKIs).