J-AXEL:在经先前治疗的NSCLC中纳布-紫杉醇 (nab-paclitaxel)与多西他赛至少相当
纳布-紫杉醇是白蛋白结合的无溶剂的紫杉醇纳米颗粒制剂,其各种优点已有描述[1-3]。II期数据显示了经过先前治疗的晚期NSCLC患者的良好结果,ORR为32%,中位PFS为5个月[4]。因此,Nakamura等人报告的随机化III期研究在先前接受过细胞毒性化疗的IIIB/IV期或复发性NSCLC患者中比较了每三周的第1、8和15天100 mg/m2的纳布-紫杉醇与每三周60 mg/m2的多西他赛[5]。 该分析旨在证明纳布-紫杉醇在OS方面的非劣效性。两组均纳入了约250名患者。
显著的PFS和ORR获益
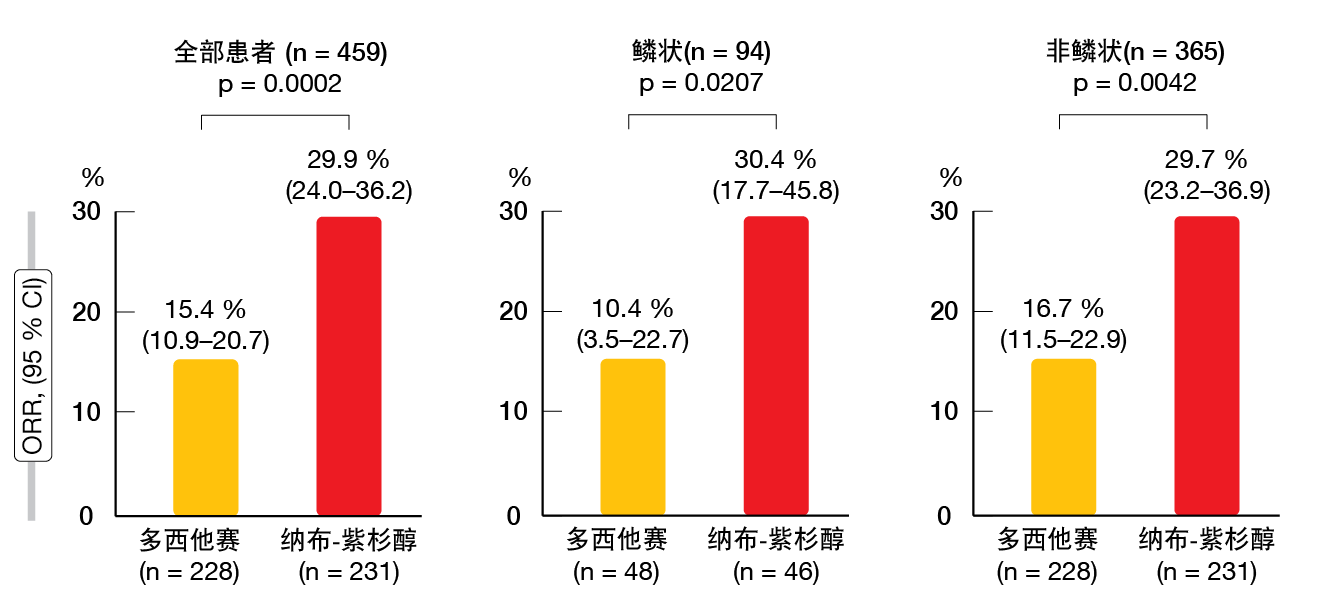
在意向治疗人群中确认了纳布-紫杉醇在OS方面的非劣效性,协议规定的界值为1.25(HR,0.85;95.2% CI,0.68–1.070)。纳布-紫杉醇和多西他赛的中位OS分别为16.2和13.6个月。按照协议规定,在显示出非劣效性之后,测试了在OS方面纳布-紫杉醇相对于多西他赛的优越性。然而纳布-紫杉醇并未显著提高生存率,PFS和ORR均是如此。纳布-紫杉醇和多西他赛的中位PFS为4.2与3.4个月(HR,0.76;p = 0.0042)。在整个组中(n = 459),对治疗有反应的患者占29.9%与15.4%(p = 0.0002;图)。对于具有鳞状组织学的患者(n = 94)为30.4%与10.4%(p = 0.0207),而对于非鳞状NSCLC的患者(n = 365)为29.7%与16.7%(p = 0.0042)。在根据不同年龄、性别、ECOG表现状态、组织学、吸烟状态、疾病阶段、EGFR突变状态和先前治疗的各个亚组中,纳布-紫杉醇的PFS和OS的结果更好。
图: 在ITT人群中并根据组织学,采用纳布-紫杉醇和多西他赛获得的客观缓解率
多西他赛的血液学毒性和纳布-紫杉醇的神经病变
在AE中,采用多西他赛的白细胞减少和中性粒细胞减少的发生率明显高于纳布-紫杉醇(两次比较均p <0.0001),相应地,多西他赛使发热性中性粒细胞减少的发生率显著增加(22.1 %与2.0%)。另一方面,采用纳布-紫杉醇的周围感觉神经病变的发生率更高(55.5%与20.1%,p <0.0001)。作者在总结中强调,对于先前接受过治疗的晚期NSCLC患者,应将纳布-紫杉醇视为标准选择。
参考文献
- Desai N et al., Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006; 12(4): 1317-1324
- Sasaki Y et al., Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci 2014; 105(7): 812-817
- Gradishar WJ et al., Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 2009; 27(22): 3611-3619
- Sakata S et al., Phase II trial of weekly nab-paclitaxel for previously treated advanced non-small cell lung cancer: Kumamoto thoracic oncology study group (KTOSG) trial 1301. Lung Cancer 2016; 99: 41-45
- Nakamura A et al., Phase III study comparing nab-paclitaxel with docetaxel in patients with previously treated advanced non-small cell lung cancer_J-AXEL. WCLC 2020, OA03.05
© 2020 Springer-Verlag GmbH, Impressum
More posts
New therapeutic options being currently investigated in advanced or metastatic colorectal cancer
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, and it is the fourth most frequent cancer diagnosis.A current treatment option for RAS and BRAF wild-type (WT) metastatic colorectal cancer (mCRC) is the chemotherapy doublet (FOLFOX/FOLFIRI) with an anti-EGFR monoclonal antibody (cetuximab or panitumumab).
An update and future directions in advanced gastric or gastrointestinal junction cancer (G/GEJC)
With more than 1 million newly diagnosed cases in 2020, gastric cancer (GC) is the fifth most frequent cancer; it was also the third leading cause of cancer-related death worldwide. Gastroesophageal junction (GEJ) cancer concerns a form of gastric cancer developing around the digestive tract where esophagus and stomach connect; in the last years, the prevalence of GEJ constantly increased.
Innovative combinations in esophageal squamous cell carcinoma
Each year, esophageal cancer (EC) is responsible for more than half a million deaths worldwide. Among them, esophageal squamous cell carcinoma (ESCC) accounts for the vast majority (~ 85 %) of EC incidences . At diagnosis, 70 % of ESCC is unresectable [3] and the 5-year survival rate is limited (30 % - 40 %). Patients with advanced or metastatic ESCC have a poor prognosis; their overall survival (OS) after standard first-line chemotherapy is limited to less than a year and other treatment options are scarce.
Novel agents or combinations in recurrent or metastatic nasopharyngeal cancer
Nasopharyngeal cancer (NPC) is a rare malignancy with an incidence of approximately 133,000 annually worldwide, resulting in about 80,000 deaths per year. Whereas early-stage and locally advanced NPC have a good prognosis, treatment of recurrent or metastatic nasopharyngeal cancer is a challenging; it is thus associated with a poor prognosis, especially in patients who have failed two or more lines of systemic therapy, with a median progression-free survival (mPFS) of seven months and median overall survival (mOS) of 22 months.
Preface ASCO Solid Tumor 2022
After 2 years of the COVID-19 pandemic, the Annual Meeting of the American Society of Clinical Oncology (ASCO), was held in Chicago, USA, and virtually from 3rd–7th June 2022.As always, the very much-anticipated event brought leading experts from across the globe together to learn and discuss the groundbreaking updates and scientific advancements which were covered in more than 2,000 abstracts, along with 85 livestream sessions, and more than 2,500 poster presentations.