Novel combination approaches in various solid tumors
Lenvatinib plus pembrolizumab
The anti-angiogenic multikinase inhibitor lenvatinib has been shown to exert immunomodulatory effects that enhance the anti-tumor activity of anti-PD-1 antibodies [1]. In the early-phase setting, lenvatinib plus pembrolizumab induced partial responses in patients with different tumor types [2]. The ongoing phase II LEAP-005 study is assessing lenvatinib 20 mg orally daily plus pembrolizumab 200 mg Q3W for up to 35 cycles in six types of pretreated, advanced solid tumors. These include triple-negative breast cancer (TNBC), ovarian cancer, gastric cancer, colorectal cancer (CRC), biliary tract cancer (BTC), and glioblastoma multiforme (GBM). ORR as well as safety and tolerability constitute the primary objectives of the study.
Lwin et al. at reported interim results for the first 187 patients enrolled in LEAP-005 at the ESMO 2020 Congress after a mean follow-up of 8.6 months [3]. For each of the tumor types assessed, 31 patients were included in the analysis with the exception of the CRC cohort that comprised 32 individuals. Notably, in this group, tumors belonged to the non-MSI-high/proficient mismatch repair category. Patients with TNBC were treated in the second or third line, those with ovarian cancer in the fourth line, those with gastric cancer and CRC in the third line and those with BTC and GBM in the second line.
Substantial disease control
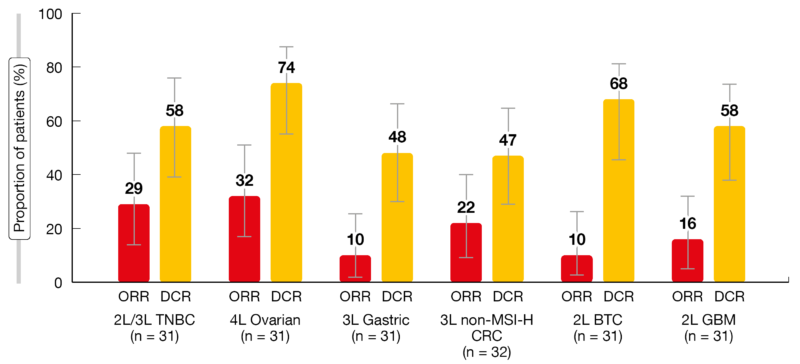
The prespecified futility efficacy criteria for cohort expansion were met or even exceeded (Figure). Regarding women’s cancers, the ORRs were 29.0 % and 32.3 % in patients with TNBC and ovarian cancer, respectively. Disease control was achieved by 58.1 % and 74.2 %, respectively. With respect to gastrointestinal cancers, ORR was highest in the CRC group (21.9 %). Both patients with gastric cancer and BTC obtained ORRs of 9.7 %. DCRs were 46.9%, 48.4 % and 67.7 %, respectively. Patients with GBM responded in 16.1 % and achieved disease control in 58.1 %. Median duration of response was 5.3 and 3.2 months for the BTC and GBM cohorts and had not been reached yet for the other cohorts.
Median PFS was longest in the BTC (median PFS, 6.1 months), ovarian cancer (4.4 months) and TNBC cohorts (4.2 months). For the other tumor types, PFS ranged between 2.3 and 2.8 months. The 6-month PFS rates in patients with TNBC and ovarian cancer were 48.9 % and 47.1 %, respectively. For gastric cancer, CRC and BTC, these were 22.2 %, 30.5 % and 56.5 %, respectively. In the GBM cohort, 11.5 % of patients were progression-free at 6 months.
Toxicity proved manageable in all cohorts. Grade 3 to 5 treatment-related AEs emerged in approximately half of patients in each cohort, although discontinuation rates due to grade 3 to 5 AEs were low at 6 % to 13 %. There was one fatal AE in each group except for the BTC cohort. Hypertension was generally the most common AE; also, fatigue, diarrhea, decreased appetite, hypothyroidism, and nausea were reported.
All-grade immune-mediated AEs occurred in 26 % to 48 %, with grade 3 to 5 events emerging in 3 % to 6 %. Infusion reactions were noted in one patient each in the TNBC, ovarian cancer and BTC cohorts. Overall, the safety profile was consistent with that previously seen with the combination of pembrolizumab and lenvatinib. LEAP-005 will continue to assess the efficacy and safety of lenvatinib plus pembrolizumab in patients with previously treated advanced solid tumors in expanded cohorts of 100 patients each.
Figure: Objective response rates (ORR) and disease control rates (DCR) across the cohorts included in the LEAP-005 trial
PD-1/PD-L1 synergy: tislelizumab and BGB-A333
Simultaneous PD-1 and PD-L1 blockade has been hypothesized to provide synergistic anti-tumor effects [4]. An open-label, phase I/IIB clinical trial evaluated the combination of the PD-1 inhibitor tislelizumab, which is currently being tested in the phase III setting, with the investigational anti-PD-L1 antibody BGB-A333 [5]. During the dose-escalation part of the study that was conducted in 15 patients, the recommended phase II dose for BGB-A333 was established at 1.350 mg intravenously Q3W. This was followed by the dose expansion phase IIB part that involved 12 patients with locally advanced or metastatic urothelial carcinoma who had progressed after at least one platinum-containing regimen. They received tislelizumab 200 mg plus BGB-A333 1,350 mg Q3W. The results obtained for this combination were presented at ESMO 2020 after a median follow-up of 10 months. Six patients each fell into the PD-L1-high and PD-L1-low categories. Four had lymph-node-only disease.
Median duration of treatment was 6.2 months. Overall, 42 % of patients responded (Table). ORR was higher in the group with PD-L1-high tumors (67 %) than in those with PD-L1-low tumors (17 %). However, given the small sample sizes, these differences should be interpreted with caution. Responses were durable and lasted for a median of 9.1 months. Median PFS amounted to 6.1 months in the total cohort; again, patients with PD-L1-high tumors fared better than those with PD-L1-low tumors (10.0 and 4.1 months, respectively).
Tislelizumab plus BGB-A333 was generally well tolerated, with most AEs showing mild or moderate severity. Fatigue constituted the most commonly reported treatment-related AE across the study. No fatal events occurred. Two patients in phase IIB experienced four immune-related AEs including grade 3 endocrine disorder, grade 3 hypophysitis, grade 2 musculoskeletal and connective tissue disorder, and grade 2 myositis. The authors noted in their conclusion that these data provide insights into combining tislelizumab with anti-PD-L1 antibody treatment.
Pamiparib plus temozolomide: biomarker analysis
Patients with various locally advanced or metastatic solid tumors are participating in the ongoing phase IB BGB-290-103 study that is evaluating the investigational PARP inhibitor pamiparib in combination with the alkylating agent temozolomide administered at low doses. A total of 114 patients were enrolled in the dose-escalation and dose-expansion phases. Most of them were heavily pretreated, with a median of 3 prior treatment lines. Pamiparib 60 mg on days 1 to 28 and temozolomide 60 mg on days 1 to 7 were identified as the recommended phase II doses.
At ESMO 2020, findings were presented from a retrospective biomarker analysis that was based on samples from patients included in both phases of the study [6]. Homologous recombination deficiency (HRD) testing was performed in archival tissue samples obtained at baseline and was expressed using the genomic instability score (GIS), which was determined based on large-scale transitions, telomeric allelic imbalance, and loss of heterozygosity. Samples with GIS ≥ 33 were defined as GIS-positive. Circulating tumor DNA next-generation sequencing was performed in blood samples obtained at baseline, with a focus on 16 core DNA damage response (DDR) genes including ATM, BRCA1, BRCA2, CDK12, PALB2, and RAD51B. A positive DDR mutational status was defined as ≥ 1 mutation in one of these 16 DDR genes. The investigators sought to establish correlations between the GIS/DDR status and overall response/disease control rates.
Robust results for GIS
Among 34 patients analyzed for HRD, 32 % were GIS-positive. These were shown to have higher ORR and DCR than GIS-negative individuals irrespective of the BRCA1/2 mutation status. For the GIS-positive cohort, ORR and DCR were 81.8 % and 90.9 %, respectively, while they were 13.0 % and 56.5 % for the GIS-negative group. In the cohort of 86 patients evaluated for DDR status, 26 % proved DDR-positive. Here, positive patients also showed higher ORR than the negative cohort (27.3 % vs. 14.1 %), although responses occurred considerably less frequently compared to the GIS-positive cohort and depended on the BRCA1/2 status. The majority of responding DDR-positive patients harbored BRCA1/2 mutations rather than the BRCA1/2 wildtype. DCRs were similar across DDR-positive and DDR-negative patients.
As the authors summarized, the GIS status, as a global measure of genomic instability, appears to be a robust biomarker for the prediction of response to pamiparib plus low-dose temozolomide. Also, this analysis confirms the observation that mutations in DDR genes other than BRCA1/2 have limited utility in predicting responses to PARP inhibitors. Cohort 6 of the study is currently evaluating the anti-tumor activity of pamiparib plus low-dose temozolomide in patients with GIS-positive NSCLC, head and neck, esophageal, and soft tissue sarcoma tumors.
REFERENCES
- Kimura T et al., Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci 2018; 109(12): 3993-4002
- Taylor M et al., A phase 1b trial of lenvatinib plus pembrolizumab in patients with selected solid tumors. Ann Oncol 2016; 27(Suppl 6): 266-295
- Lwin Z et al., LEAP-005: phase 2 study of lenvatinib plus pembrolizumab in patients with previously treated advanced solid tumors. ESMO 2020, LBA41
- Naing A et al., Anti-PD-1 monoclonal antibody MEDI0680 in a phase I study of patients with advanced solid malignancies. Immunother Cancer 2019; 7(1): 225
- Martin-Liberal J et al., BGB-A333, an anti-PD-l1 monoclonal antibody, in combination with tislelizumab in patients with urothelial carcinoma. ESMO 2020, 535MO
- Stradella A et al., Clinical benefit in biomarker-positive patients with locally advanced or metastatic solid tumors treated with the PARP1/2 inhibitor pamiparib in combination with low-dose temozolomide. ESMO 2010, 530MO
© 2020 Springer-Verlag GmbH, Impressum