COVID-19 in patients with thoracic cancers: TERAVOLT
The global consortium TERAVOLT was established to determine factors that place patients with thoracic malignancies who develop COVID-19 at risk for hospitalization and death, to elucidate the clinical course of these patients and to identify therapeutic strategies that might impact survival. Thoracic cancer patients with a COVID-19 diagnosis, i.e. cases of confirmed infection according to RT-PCR techniques and suspected COVID-19 cases, are being entered into the database. The latter are defined by either clinical criteria (known exposure to a person with confirmed COVID-19 and symptoms such as fever > 37.5 °C, cough, diarrhea etc.) or lung imaging features consistent with coronavirus pneumonia and symptoms.
The analysis presented at ASCO 2020 included a global population of 400 patients with a median follow-up of 33 days from the COVID-19 diagnosis [1]. At the time of data cut-off, 169 of patients had recovered, while 141 had died (35.5 %) and the infection was ongoing in 118. The median age across these groups ranged from 67 to 70 years. Most patients were male and current or former smokers.
Chemotherapy increases mortality
Presenting COVID-19 symptoms mainly included fever, cough, and dyspnea. In 78.3 % and 8.3 %, hospital and ICU admissions, respectively, became necessary. The median length of stay at hospital was 10 days. Among the patients who died, COVID-19 was the cause of death in 79.4 %, while only 10.6 % of fatalities were attributed to cancer. The most common complications in the deceased group comprised pneumonitis/pneumonia (71.0 %), acute respiratory distress syndrome (49.6 %), multiorgan failure (14.9 %), and sepsis (12.1 %).
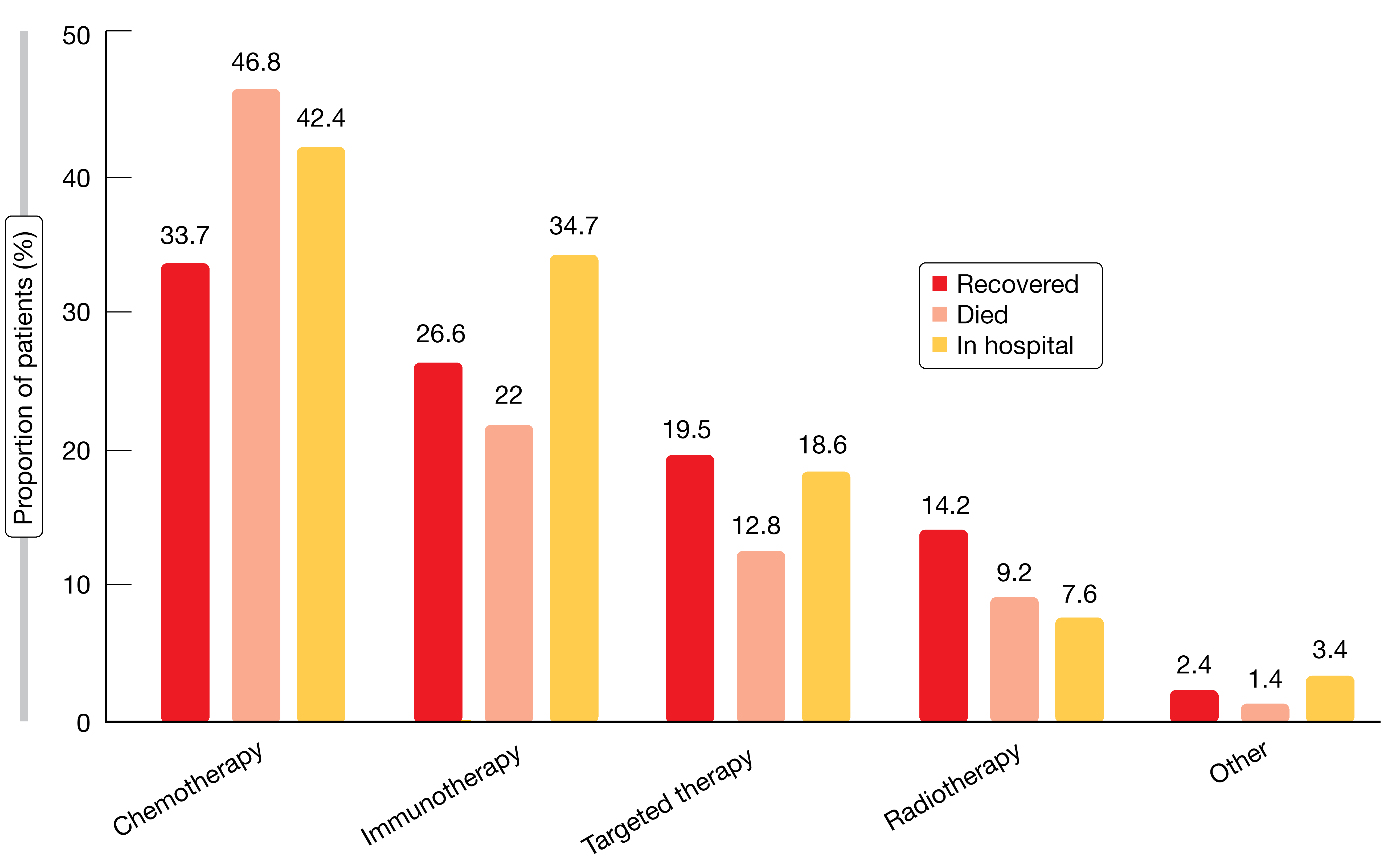
Baseline risk factors for mortality from COVID-19 included age ≥ 65 years, performance status of 1 and presence of comorbidities (e.g., hypertension, COPD, vascular disease), while other factors such as gender, body mass index, smoking status, and stage or type of cancer did not affect the risk of death. Steroids (> 10 mg of prednisone or equivalent) or anticoagulation prior to the diagnosis of COVID-19 increased the risk, as did prior administration of chemotherapy alone or combined with immunotherapy, while immunotherapy and TKI treatment had no adverse effect on survival (Figure). No particular treatment for COVID-19 was associated with increased chances of recovery from the infection. Data collection is ongoing, and additional analyses are planned.
REFERENCES
- Horn L et al., TERAVOLT: Thoracic cancERs international coVid 19 COLlaboraTion: impact of cancer therapy and COVID therapy on survival. J Clin Oncol 38: 2020 (suppl; abstr LBA111)
More posts
Rare mutations: HER2, RET, ALK, BRAF
Trastuzumab deruxtecan (T-DXd) is a novel antibody-drug conjugate containing a humanized anti-HER2 monoclonal antibody linked to a topoisomerase I inhibitor exatecan derivative. The open-label, multicenter, phase II DESTINY-Lung01 study tested T-DXd 6.4 mg/kg 3-weekly in patients with relapsed or refractory advanced NSCLC that expressed HER2 (Cohort 1; n = 42) or carried HER2-activating mutations (Cohort 2; n = 42).
COVID-19 in patients with thoracic cancers: TERAVOLT
The global consortium TERAVOLT was established to determine factors that place patients with thoracic malignancies who develop COVID-19 at risk for hospitalization and death, to elucidate the clinical course of these patients and to identify therapeutic strategies that might impact survival. Thoracic cancer patients with a COVID-19 diagnosis, i.e. cases of confirmed infection according to RT-PCR techniques and suspected COVID-19 cases, are being entered into the database.
Present and future perspectives of anti-angiogenic therapy
The oral, triple angiokinase inhibitor nintedanib has been approved in the European Union and other countries in combination with docetaxel for the treatment of advanced adenocarcinoma of the lung after first-line chemotherapy. It works by targeting vascular endothelial growth factor (VEGF) receptors 1-3, platelet-derived growth factor (PDGF) receptors α/β and fibroblast growth factor (FGF) receptors 1-3, as well as RET.
Improving outcomes in the early-stage setting with (neo)adjuvant strategies
Approximately 30 % of NSCLC patients present with resectable disease at diagnosis. Surgery is the primary treatment for early-stage NSCLC; after resection, adjuvant cisplatin-based chemotherapy is recommended for patients with stage II/IIIA lung cancer and select patients with stage IB disease. However, the rates for disease recurrence or death following surgery and adjuvant chemotherapy remain high, ranging from 45 % in stage IB to 76 % in stage III.
EGFR-mutated disease: early combinations and new approaches in exon 20 insertion-positive lung cancer
Oligometastatic disease is generally defined by one to five metastatic lesions. As progression occurs most frequently in sites of the original disease, it is surmised that aggressive local treatment might prevent further dissemination. Based on this rationale, the open-label, randomized, phase III SINDAS trial conducted in China explored the use of concurrent stereotactic body radiotherapy (SBRT) and EGFR TKI therapy in patients with oligometastatic, EGFR-mutant NSCLC.
Immune checkpoint inhibition: comprehensive benefits, but not devoid of risks
First-line nivolumab plus ipilimumab (NI) was shown to significantly prolong OS compared to chemotherapy in patients with advanced NSCLC irrespective of tumor PD-L1 expression in the randomized, phase III CheckMate 227 study. At the ASCO Congress, Ramalingam et al. presented the updated 3-year efficacy and safety results from Part 1 of the trial.