Rare mutations: HER2, RET, ALK, BRAF
DESTINY-Lung01: trastuzumab deruxtecan
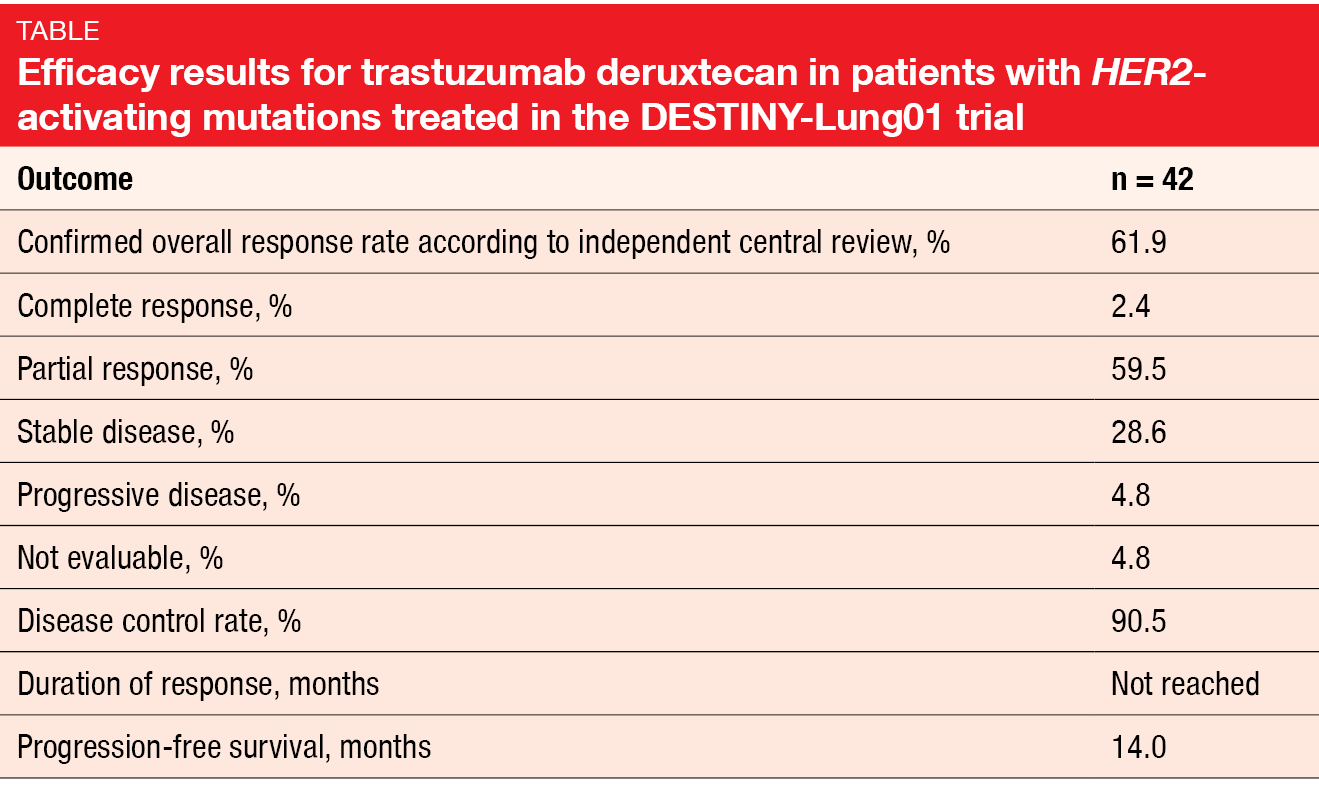
Trastuzumab deruxtecan (T-DXd) is a novel antibody-drug conjugate containing a humanized anti-HER2 monoclonal antibody linked to a topoisomerase I inhibitor exatecan derivative. The open-label, multicenter, phase II DESTINY-Lung01 study tested T-DXd 6.4 mg/kg 3-weekly in patients with relapsed or refractory advanced NSCLC that expressed HER2 (Cohort 1; n = 42) or carried HER2-activating mutations (Cohort 2; n = 42). At the ASCO Congress, Smit et al. reported the interim results for Cohort 2 [1]. In terms of confirmed ORR according to independent central review, which was the primary endpoint, T-DXd demonstrated pronounced clinical activity. Almost 62 % of patients responded, and 2.4 % achieved complete remissions (Table). At the time of the analysis, the median duration of response had not been reached yet; this also applied to OS. Median PFS was 14.0 months.
The safety profile observed in this HER2-mutated cohort was generally consistent with previously reports. Nausea, alopecia, anemia, neutropenia and decreased appetite represented the most common treatment-emergent AEs. Fatigue and nausea primarily prompted dose reductions, while dose interruptions were predominantly due to neutropenia (19.0 %) and lung infection (7.1 %). Five patients developed grade-2 interstitial lung disease (ILD). The authors noted that ILD remains a concern and requires careful monitoring and management. Overall, these data show the potential of T-DXd as a new treatment option in the setting of HER2-mutated NSCLC. Meanwhile, enrolment in the HER2-mutated cohort has been expanded to better characterize the risk-benefit ratio of T-DXd.
CNS effects of selpercatinib in RET-positive lung cancer
RET fusions have been identified in approximately 2 % of NSCLC patients [2, 3]. The ongoing registrational, international, phase I/II LIBRETTO-001 trial is assessing the efficacy of the selective, CNS-active RET inhibitor selpercatinib (LOXO-292). LIBRETTO-001 is being conducted in patients with advanced RET-fusion–positive solid tumors; 253 of these have NSCLC. In the primary analysis set, the ORR was 68 %, and responses lasted for a median of 20.3 months [4].
Data from the NSCLC CNS population with measurable CNS disease (n = 22) presented by Subbiah et al. shed more light on the intracranial activity of selpercatinib [5]. Overall, the CNS ORR was 81.8 %, with CRs occurring in 22.7 %. Patients without prior irradiation to the brain fared somewhat better than those who had received radiotherapy (ORR, 85.7 % vs. 75.0 %; CR, 28.6 % vs. 12. 5%). Both patients with and without prior anti-PD-(L)1 treatment responded intracranially; also, this was not affected by the prior use of multi-targeted kinase inhibitor therapy. Median duration of CNS response was 9.4 in the total group. The authors concluded that selpercatinib shows marked and durable intracranial anti-tumor activity in patients with RET-fusion–positive NSCLC and CNS metastases. A randomized, global, phase III study of selpercatinib versus platinum-based chemotherapy with or without pembrolizumab in treatment-naïve RET-fusion–positive NSCLC including patients with asymptomatic brain metastases is ongoing.
RET kinase inhibitor pralisetinib
Another investigational, selective RET kinase inhibitor that is currently being developed is pralisetinib (BLU-667). The ongoing pivotal, global, phase I/II ARROW trial is testing pralisetinib in patients with advanced solid, RET-altered tumors including RET-fusion–positive NSCLC. Gainor et al. reported data for the intent-to-treat (ITT) efficacy population of 132 NSCLC patients that included 116 response-evaluable individuals [6]. In the ITT population, 92 and 29 patients had received prior platinum and were treatment-naïve, respectively.
Pralsetinib gave rise to rapid and durable responses. According to blinded independent centralized review, the ORR in the response-evaluable group was 65 %, with 6 % obtaining CRs. Disease control resulted in 93 %. The median duration of response had not been reached yet. All treatment-naïve patients in the evaluable cohort achieved tumor reductions, and 12 % experienced CRs. Furthermore, pralisetinib showed robust activity in the CNS, with intracranial ORR and CR rates of 56 % and 33 %, respectively.
The RET inhibitor therapy was well tolerated. Treatment-related AEs included mainly transaminase elevations, cytopenia, constipation and hypertension, and were predominantly grades 1 and 2. In their summary, the authors emphasized that pralisetinib has the potential to change the standard of care for patients with RET-fusion–positive NSCLC.
Survival update of ALEX
Previous analyses of the global, randomized, phase III ALEX study have established the superiority of alectinib over crizotinib in patients with untreated, advanced ALK-positive NSCLC. The mature PFS data confirmed significant improvement for this endpoint (34.8 vs. 10.9 months) [7], while the OS results remained immature. After a further 12 months of follow-up, Peters et al. presented updated OS and other outcomes [8].
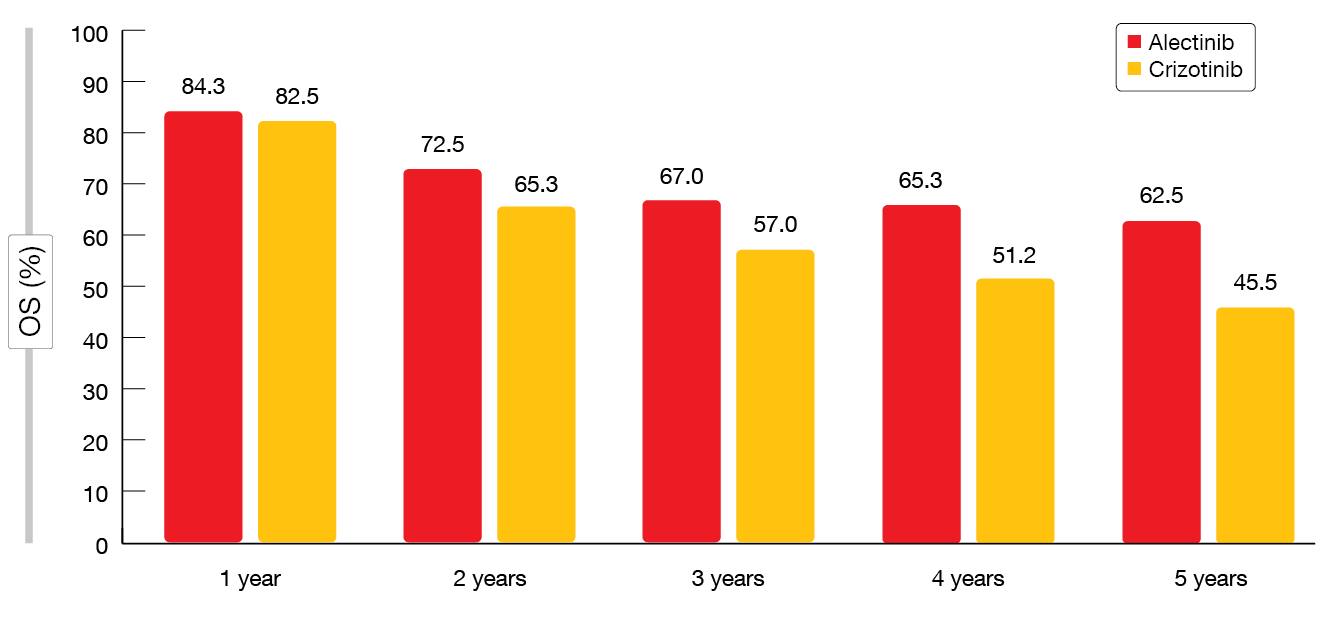
The OS data were still immature at that time, with five-year OS rates of 62.5 % vs. 45.5 % for alectinib and crizotinib, respectively (HR, 0.67; p = 0.0376; Figure). Among patients who experienced disease progression, subsequent therapy was administered in more than 60 % in both arms. Follow-up treatment with other ALK TKIs was prescribed to 38.1 % and 53.5 % of alectinib- and crizotinib-treated patients with progressive disease. No new safety signals occurred after almost three-times longer median treatment duration with alectinib (28.1 months) than crizotinib (10.8 months). The investigators concluded that ALEX is the first global, randomized trial of a next-generation ALK TKI to demonstrate a clinically meaningful OS improvement compared to crizotinib in treatment-naïve, advanced ALK-positive NSCLC.
Figure: Overall survival rates obtained in the ALEX study with alectinib and crizotinib over time
Impact of biomarkers in ALTA-1L
The open-label, randomized, multicenter, phase III ALTA-1L study evaluated brigatinib in patients with ALK-TKI–naïve, ALK-positive advanced NSCLC, demonstrating superior efficacy compared to crizotinib with acceptable tolerability at the second interim analysis [9]. Camidge et al. evaluated the impact of EML4-ALK fusion variants and other baseline variables on the activity of brigatinib vs. crizotinib in the ALTA-1L trial [10].
Brigatinib was superior to crizotinib with respect to ORR and PFS regardless of EML4-ALK fusion variant or TP53 mutation status. EML4-ALK fusion variant 3 (V3) appeared to be prognostic, as patients with this variant had worse PFS than those harboring V1 or V2, irrespective of treatment. Brigatinib demonstrated superior PFS particularly in this poor-prognosis group of patients with V3 (HR, 0.30). Also, there was a trend indicating that TP53 mutations are an independent biomarker of poor prognosis which persisted in multivariate analyses and warrants further investigation in a larger sample size. Defining higher-risk ALK-positive advanced NSCLC might impact future clinical trial designs and treatment options.
BRAFV600E-mutant disease: dabrafenib & trametinib
Dabrafenib as a monotherapy and combined with trametinib was assessed in a non-randomized, multicenter, open-label phase II trial in patients with BRAFV600E-mutant metastatic NSCLC. The primary analysis has revealed robust clinical activity for dabrafenib plus trametinib with a manageable safety profile [11]. At the ASCO Congress, Planchard et al. presented the updated OS and genomic analysis data for the combination therapy population in Cohorts B (pretreated patients) and C (treatment-naïve patients) [12].
Dabrafenib plus trametinib provided combined CR and PR rates of 68.4 % and 63.9 % in Cohorts B and C, respectively. Reponses lasted for 9.8 and 10.2 months, respectively. OS was 18.2 months in Cohort B and 17.3 months in Cohort 3. The genomic analysis suggested that co-occurring genetic alterations influence clinical outcomes, as patients with PI3K pathway alterations showed a trend towards decreased OS. Toxicities of the combined treatment were manageable; the safety profile matched that reported for patients with melanoma who receive dabrafenib and trametinib. Overall, the combination provided durable clinical benefit with a favorable risk/benefit ratio regardless of prior treatment.
REFERENCES
- Smit E et al., Trastuzumab deruxtecan in patients with HER2-mutated metastatic non-small cell lung cancer: interim results of DESTINY-Lung01. J Clin Oncol 38: 2020 (suppl; abstr 9504)
- Takeuchi K, Discovery stories of RET fusions in lung cancer: a mini-review. Frontiers Physiol 2019; 10: 216
- Tsuta K et al., RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Brit J Cancer 2014; 110(6): 1571-1578
- Drilon A et al., Registrational results of LIBRETTO-001: a phase 1/2 trial of selpercatinib (LOXO-292) in patients with RET-fusion–positive lung cancer. WCLC 2019, abstract #PL02.08
- Subbiah V et al., Intracranial activity of selpercatinib (LOXO-292) in RET fusion-positive non-small cell lung cancer (NSCLC) patients on the LIBRETTO-001 trial. J Clin Oncol 38: 2020 (suppl; abstr 9516)
- Gainor JF et al., Registrational dataset from the phase 1/2 ARROW trial of pralisetinib (BLU-667) in patients with advanced RET fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol 38: 2020 (suppl; abstr 9515)
- Mok T et al., Updated overall survival and final progression-free survival data for patients with treatment-naïve advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020; S0923-7534(20)39796-9
- Peters S et al., Updated overall survival and safety data from the randomized, phase III ALEX study of alectinib vs crizotinib in untreated advanced ALK+ NSCLC. J Clin Oncol 38: 2020 (suppl; abstr 9518
- Camidge DR et al., Brigatinib vs. crizotinib in patents with ALK inhibitor-naïve advanced ALK+ NSCLC: updated results from the phase III ALTA-1L trial. Ann Oncol 2019; 30 (suppl_9): ix183-ix202
- Camidge DR et al., Correlation of baseline molecular and clinical variables with ALK inhibitor efficacy in ALTA-1L. J Clin Oncol 38: 2020 (suppl; abstr 9517)
- Planchard D et al., Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017; 18(10): 1307-1316
- Planchard D et al., The updated overall survival and genomic analysis from a single-arm phase 2 study of dabrafenib plus trametinib in patients with BRAFV600E mutant metastatic non-small cell lung cancer. J Clin Oncol 38: 2020 (suppl; abstr 9593)