Emerging survival benefits in the small-cell setting
IMpower133: updated OS results
Given the dismal prognosis of patients with extensive-stage small-cell lung cancer (ES-SCLC), there is a high need of effective first-line treatment options. The global, double-blind, randomized, placebo-controlled, phase I/III IMpower133 study was the first trial to demonstrate survival benefits in ES-SCLC with the PD-L1 inhibitor atezolizumab plus carboplatin and etoposide compared to placebo plus chemotherapy [1]. Median OS was 12.3 vs. 10.3 months in the two treatment arms (HR, 0.70; p = 0.007), along with a tolerable safety profile. Based on these results, atezolizumab plus carboplatin and etoposide received approval for the first-line treatment of patients with ES-SCLC.
Reck et al. presented updated OS findings of IMpower133 after an additional follow-up of 9 months (median follow-up, 22.9 months) [2]. In the ITT population, atezolizumab plus chemotherapy gave rise to continuous OS improvement (median, 12.3 vs. 10.3 months; HR, 0.76; p = 0.0154). The landmark analysis at 18 months demonstrated a survival advantage of 13 % in the experimental arm (34.0 % vs. 21.0 %). According to the conclusion of the authors, these results further support the combination of atezolizumab with chemotherapy as the new standard of care for untreated ES-SCLC in an all-comer patient population.
Additional analyses of CASPIAN and IMpower133
Similarly, the global, randomized, open-label, phase III CASPIAN study has shown statistically significant improvement in OS with first-line durvalumab plus etoposide plus platinum-based chemotherapy (EP) versus EP alone in patients with treatment-naïve ES-SCLC (median OS, 13.0 vs. 10.3 months; HR, 0.73; p = 0.0047) [3]. No additional toxicity was noted. At ESMO 2019, Paz-Ares et al. reported patterns of first progression and patient-reported outcomes in the CASPIAN trial [4]. The analysis indicated that numerically fewer patients in the durvalumab-treated arm developed new lesions at first progression compared to the control arm (41.4 % vs. 47.2 %). However, no difference was found for new brain/CNS lesions (11.6 % vs. 11.5 %). In line with the efficacy outcomes, all patient-reported outcomes including time to deterioration for all symptoms, functioning, and health-related quality of life favored durvalumab plus EP compared with EP alone.
Both IMpower133 and CASPIAN analyzed survival based on biomarker expression. The exploratory analysis of IMpower133, which included PD-L1 immunohistochemistry and assessment of blood tumor mutational burden, suggested that patients derive OS benefit from the addition of atezolizumab regardless of biomarker status [2]. However, PD-L1 assessments were based on a limited data set, as only 34 % of the ITT population were PD-L1–evaluable. Likewise, CASPIAN showed no significant interaction of PD-L1 expression with clinical outcomes [4]. The PD-L1 status was evaluable in 51.6 % of patients. PD-L1 expression was low, with 94.9 % and 77.6 % showing levels < 1 % on tumor cells and immune cells, respectively. The authors of both trials concluded that further analyses are needed to evaluate the association of potential biomarkers with clinical outcomes for ES-SCLC patients.
Third line and later lines: anlotinib
The multi-targeted TKI anlotinib works by selectively inhibiting various growth factor receptors that enhance proangiogenic pathways as well as tumor proliferation and are expressed at increased levels in SCLC. The multicenter, randomized, double-blind, phase II ALTER1202 trial assessed anlotinib in patients with limited-stage SCLC or ES-SCLC who had developed progression after ≥ 2 lines of chemotherapy. A previous analysis has shown significantly improved PFS compared to placebo (4.1 vs. 0.7 months; HR, 0.19; p < 0.0001) [5]. At that time, findings with respect to survival had been immature.
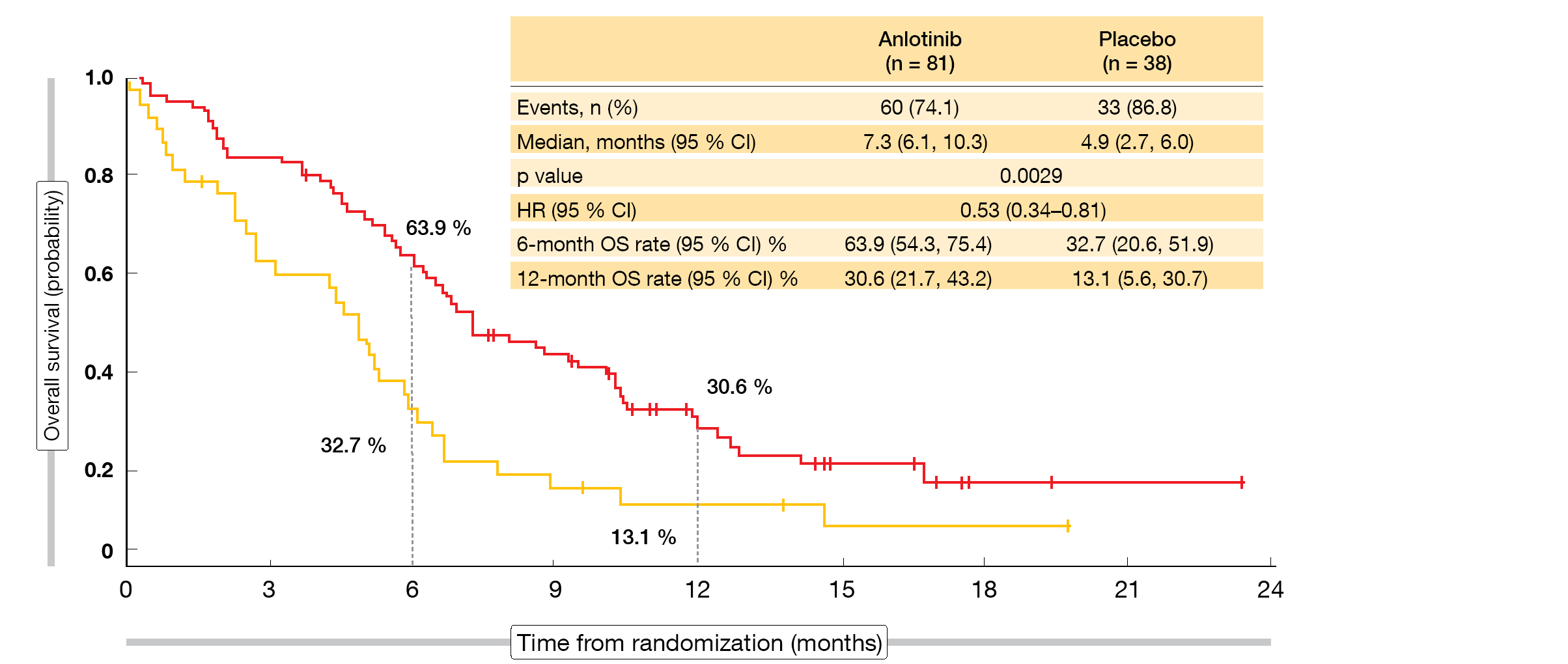
According to the updated OS results presented at ESMO 2019, anlotinib was significantly superior to placebo, with median OS of 7.3 vs. 4.9 months (HR, 0.53; p = 0.0029; Figure) [6]. Survival rates with anlotinib exceeded those observed for placebo at 6 months (63.9 % vs. 32.7 %) and 12 months (30.6% vs. 13.1 %). Most of the subgroups benefited from the active treatment. Patients with brain metastases showed a 77 % reduction in mortality (median OS, 6.3 vs. 2.6 months; HR, 0.23; p = 0.0009). In this cohort, 55.7 % vs. 0 % were alive at 6 months.
ALTER1202 is the first randomized, double-blind study to demonstrate a survival advantage for patients with relapsed SCLC who have experienced treatment failure after at least two treatment lines. The authors suggested that anlotinib should be considered a new standard of care for patients with SCLC progressing after second-line or later-line chemotherapy.
Figure: ALTER1202: overall survival advantage with anlotinib over placebo in patients treated with two or more previous lines of chemotherapy
REFERENCES
- Horn L et al., First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220-2229
- Reck M et al., IMPOWER133: Updated overall survival analysis of first line atezolizumab + carboplatin + etoposide in extensive stage SCLC. ESMO 2019, abstract 1736O
- Paz Ares L, et al. Overall survival with durvalumab plus etoposide-platinum in first-line extensive-stage SCLC: results from the CASPIAN study. WCLC 2019, abstract PL02.11
- Garassino MC et al., PD-L1 expression, patterns of progression and patients reported outcomes with durvalumab plus platinum etoposide in ES SCLC: results from CASPIAN. ESMO 2019, abstract LBA89
- Cheng Y et al., Anlotinib as third-line or further-line treatment in relapsed SCLC: a multicenter, randomized, double-blind phase 2 trial. J Thorac Oncol 2018; 13(10): S351-S352
- Cheng Y et al., Overall survival update in ALTER 1202: anlotinib as third line or further line treatment in relapsed SCLC. ESMO 2019, abstract 1738O