Exploring synergy between anti-angiogenic drugs and immunotherapy
In the setting of non-squamous advanced NSCLC without actionable driver mutations, the advent of immune checkpoint inhibitor therapy has led to the implementation of new standards. Synergistic effects can be expected from anti-angiogenic treatment. The vascular endothelial growth factor (VEGF) has been shown to create an immunosuppressive tumor microenvironment by modifying immune cell function besides promoting angiogenesis [1-3]. These mechanisms are likely to contribute to immune checkpoint inhibitor resistance but can be antagonized using agents such as the triple angiokinase inhibitor nintedanib. The angio-immunogenic switch describes the restitution of an immunosupportive tumor microenvironment based on vessel normalization and improved access of immune cells to the tissue [4].
VARGADO: nintedanib after immunotherapy
Evidence that might provide guidance regarding the selection of treatment after progression on immune checkpoint inhibitor therapy has been obtained from the German non-interventional, prospective VARGADO trial. VARGADO is evaluating nintedanib plus docetaxel after first-line chemotherapy in routine care. The study comprises three cohorts, among them Cohort B in which patients are treated with frontline chemotherapy followed by immune checkpoint inhibition in the second line and nintedanib/docetaxel in the third. Grohé et al. presented data from an updated interim analysis of Cohort B (n = 32) at ESMO 2019 [5].
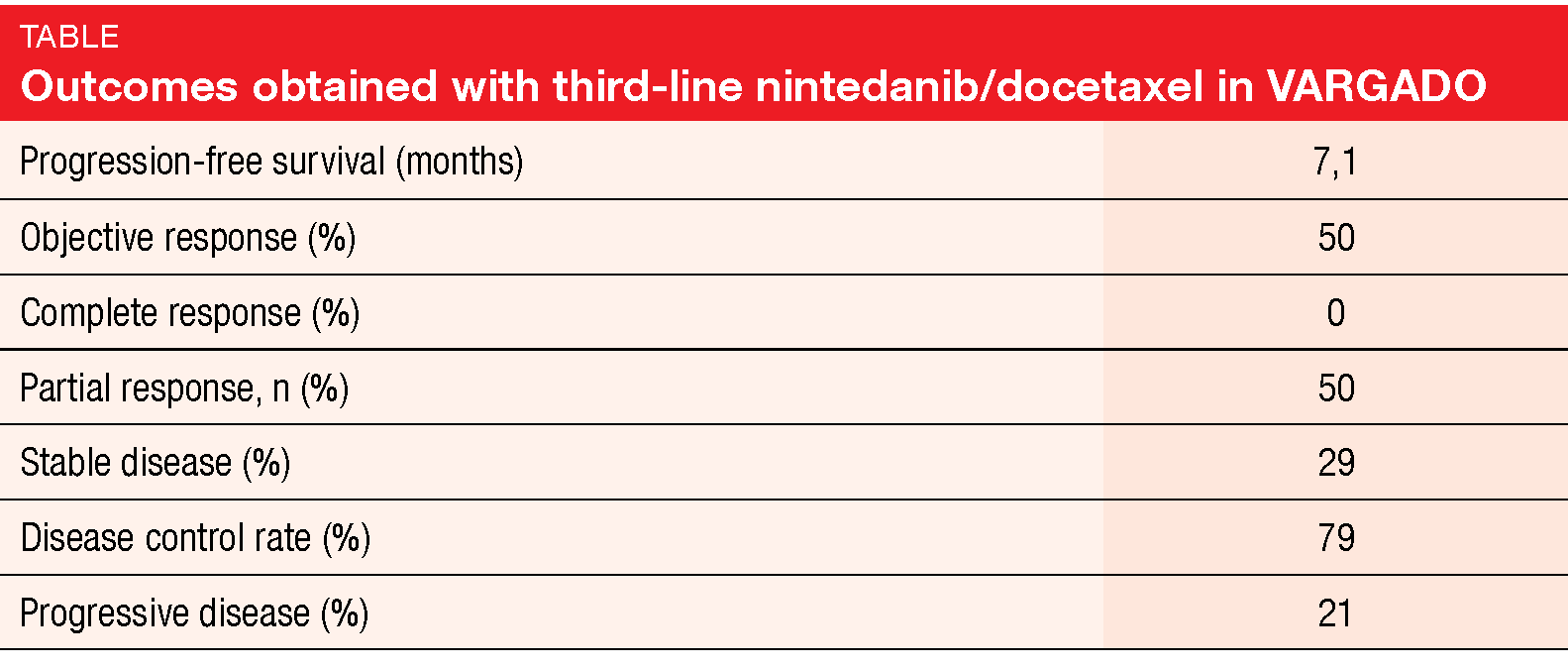
After a median follow-up of 6.9 months for nintedanib/docetaxel, median PFS was 7.1 months in the third-line setting. Best overall response data were available for 24 patients, with partial responses occurring in 50 % (Table). The disease control rate was 79 %. Overall, this updated analysis of VARGADO continued to demonstrate the clinical benefit of nintedanib plus docetaxel in patients who progressed on immunotherapy. Overall survival data are not mature yet. The authors noted that rational sequencing of an anti-angiogenic agent after immune checkpoint inhibition might be a promising treatment approach in this patient population that warrants further investigation.
SENECA: immunotherapy after nintedanib
A similar setup was analyzed based on data obtained by the open-label, phase IIb SENECA trial. This Italian real-world study tested two docetaxel schedules (33 mg/m2 on days 1 and 8 three-weekly; 75 mg/m2 on day 1 three-weekly) along with continuous oral nintedanib in patients with advanced non-squamous NSCLC who had progressed after first-line chemotherapy. Nintedanib maintenance was administered in case of disease control. The final analysis (n = 170) confirmed the efficacy of second-line nintedanib/docetaxel irrespective of the duration of the relapse-free interval after first-line chemotherapy and the docetaxel schedule, with similar OS and PFS findings in both dosing groups [6].
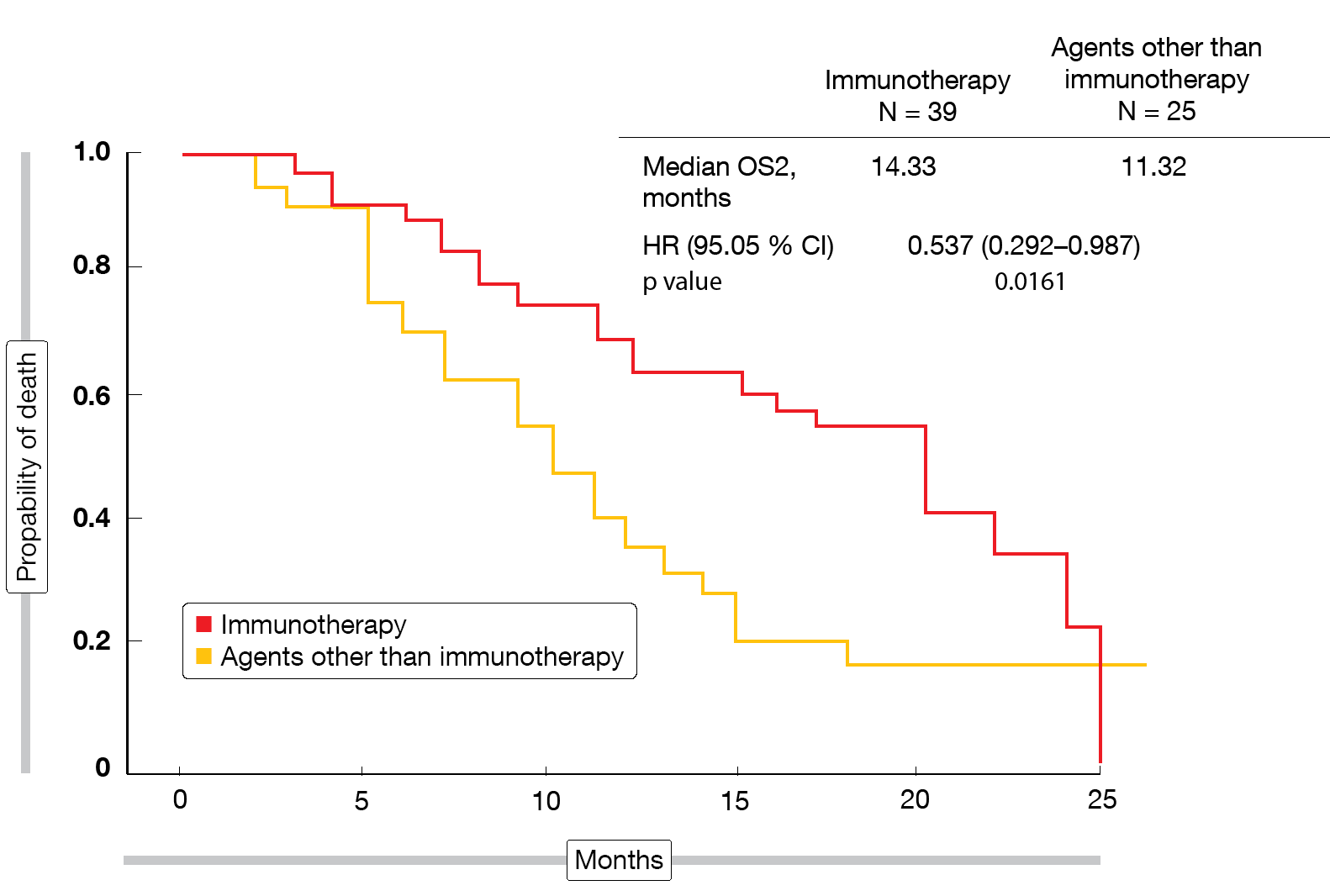
The data presented at ESMO 2019 focused on the effects of second-line nintedanib/docetaxel on subsequent immunotherapy in SENECA [7]. Post-progression outcomes were assessed in 64 patients (37.6 % of the entire study population) for those who received third-line treatment with immune checkpoint inhibitors (nivolumab, atezolizumab, pembrolizumab; n = 39) versus those treated with other third-line agents (gemcitabine, vinorelbine, erlotinib, crizotinib, cabozantinib; n = 25). The primary endpoints were PFS2 and OS2, the latter of which was defined as the time from the start of docetaxel/nintedanib treatment to progression during third-line therapy or death. According to the analysis, PFS2 did not differ between patients who received immunotherapy and those who did not (10.78 vs. 7.91 months; HR, 0.602; p = 0.0821). However, for OS2, the immunotherapy-treated group fared significantly better (14.33 vs. 11.32 months; HR, 0.537; p = 0.0161; Figure). As the authors pointed out, the survival benefit observed with the sequence of docetaxel/nintedanib and immunotherapy became apparent despite the small sample size of this analysis. The synergism between antiangiogenic agents and immunotherapy might be an attractive basis for the development of new therapeutic algorithms.
Figure: Time from initiation of docetaxel/nintedanib to progression or death during third-line treatment with immunotherapy or other agents
REFERENCES
- Fukumura D et al., Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018; 15(5): 325-340
- Khan KA & Kerbel RS, Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 2018; 15(5): 310-324
- Chouaib S et al., Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol 2012; 3: 21
- Grohé C et al., Nintedanib plus docetaxel in lung adenocarcinoma patients following treatment with immune checkpoint inhibitors: Preliminary efficacy and safety results of the non-interventional study VARGADO. J Clin Oncol 37, 2019 (suppl; abstr 9074)
- Grohé C et al., Efficacy and safety of nintedanib plus docetaxel in lung adenocarcinoma patients following treatment with immune checkpoint inhibitors: updated results of the ongoing non-interventional study VARGADO (NCT02392455). ESMO 2019, abstract 1505P
- Capelletto E et al., Final results of the SENECA (SEcond line NintEdanib in non-small cell lung CAncer) trial. Lung Cancer 2019; 134: 210-217
- Capelletto E et al., Post-progression survival for patients treated with docetaxel/nintedanib in the SENECA trial. ESMO 2019, abstract 1568P