Improving outcomes in the early-stage setting with (neo)adjuvant strategies
Approximately 30 % of NSCLC patients present with resectable disease at diagnosis [1-3]. Surgery is the primary treatment for early-stage NSCLC; after resection, adjuvant cisplatin-based chemotherapy is recommended for patients with stage II/IIIA lung cancer and select patients with stage IB disease [4]. However, the rates for disease recurrence or death following surgery and adjuvant chemotherapy remain high, ranging from 45 % in stage IB to 76 % in stage III [5]. Clearly, there is an unmet need for novel and effective therapies to improve clinical outcomes.
ADAURA: adjuvant use of osimertinib
The third-generation EGFR TKI osimertinib has been established as a standard-of-care first- and second-line treatment option in patients with EGFR-mutant advanced NSCLC. Based on the observation that the efficacy and safety profile of this agent suggest activity in early-stage disease [6], the double-blind, randomized, phase III ADAURA trial compared osimertinib 80 mg daily (n = 339) with placebo (n = 343) in patients who had undergone complete resection of EGFR-mutant (i.e., exon 19 deletion or L858R mutation), non-squamous lung cancer. Histology had shown negative resection margins, and imaging including brain CT or MRI scans demonstrated the absence of disease. Delivery of post-operative standard adjuvant chemotherapy was allowed prior to randomization, while radiotherapy was not. The maximum interval between surgery and randomization comprised 10 or 26 weeks without or with adjuvant chemotherapy, respectively. The planned treatment duration was three years. Approximately one third of patients each belonged to stages IB, II, and IIIA in both arms, and 55 % had received adjuvant chemotherapy. Disease-free survival in stage II/IIIA patients was defined as the primary endpoint.
Following a recommendation by the independent data monitoring committee, the study was unblinded two years early due to an overwhelming benefit of the osimertinib treatment. At the ASCO Congress, Herbst et al. reported an unplanned interim analysis of the ADAURA trial [7]. At the time of unblinding, the study had completed enrollment, and all patients had been followed up for at least one year.
Substantial risk reductions
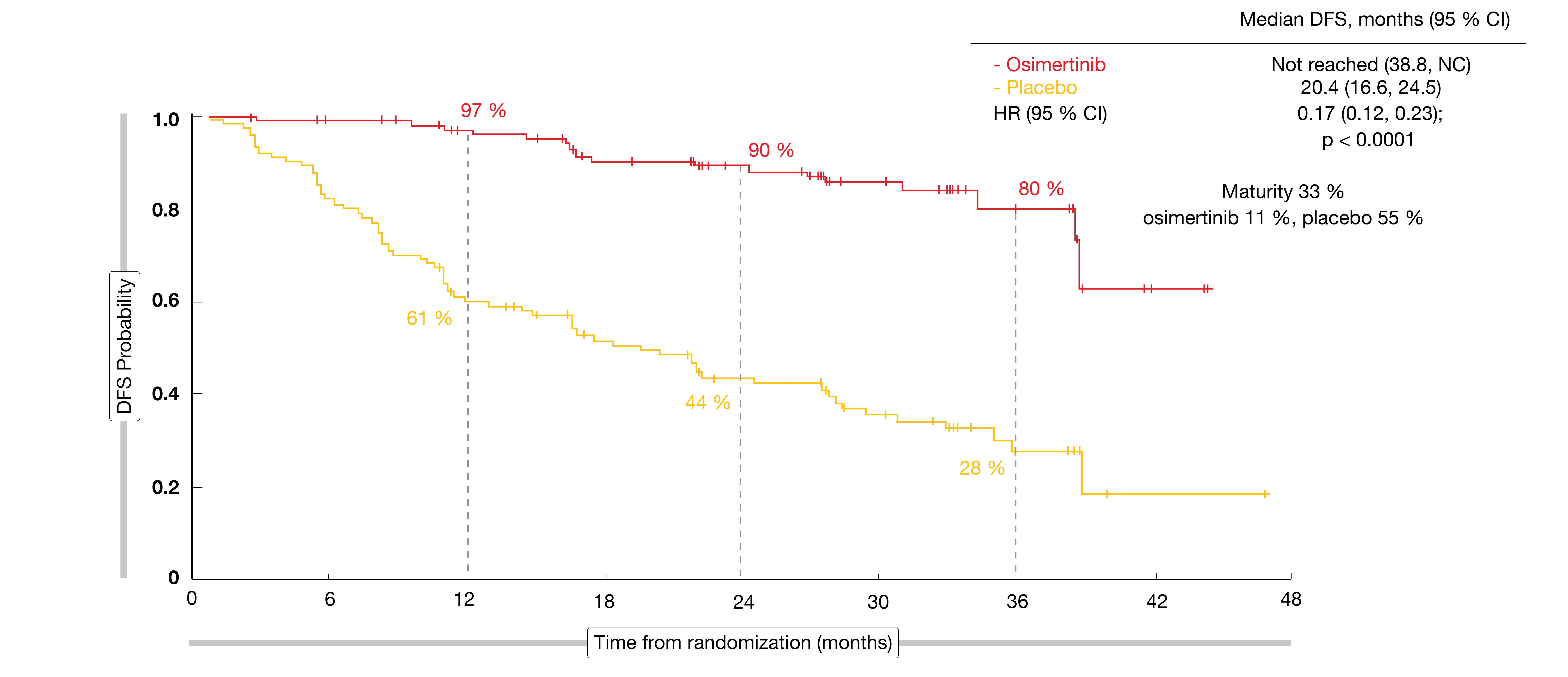
For the primary endpoint, osimertinib treatment induced an 83 % reduction in the risk of disease recurrence or death in patients with stage II/IIIA disease. Median DFS had not been reached in the experimental arm and was 20.4 months in the control arm (HR, 0.17; p < 0.0001; Figure 1). The key secondary endpoint of DFS in the overall population was also met. Even with the addition of lower-risk patients with stage IB disease, the risk reduction amounted to 79 % (not reached vs. 28.1 months; HR, 0.21; p < 0.0001). At two years, DFS rates were 89 % vs. 53 %, respectively.
All of the pre-specified subgroups benefited from osimertinib treatment; notably, DFS was improved regardless of whether patients had received prior adjuvant chemotherapy. An analysis conducted according to disease stage showed that in the osimertinib arm, 2-year DFS rates remained high across stages IB (87 %), II (91 %) and IIIA (88 %), whereas they decreased rapidly in the placebo arm with increasing stage. The hazard ratios therefore indicated the greatest risk reductions in stages II (0.17) and IIIA (0.12). OS results were immature, but the interim analysis already suggested a 60 % benefit (HR, 0.40). The safety profile of adjuvant osimertinib matched the established safety profile. Diarrhea occurred as the most common AE in 46 % of patients, followed by paronychia (25 %) and dry skin (23 %). AEs were generally mild. Grade-1/2 interstitial lung disease was reported in 10 osimertinib-treated patients (3 %), and QTc prolongation emerged in 22 (7 %) vs. 4 (1 %) patients.
In their summary, the authors pointed out that adjuvant osimertinib is the first targeted agent in a global trial to show a statistically significant and clinically meaningful improvement in DFS in patients with stage IB/II/IIIA, EGFR-mutated NSCLC. Osimertinib therefore represents a highly effective, practice-changing treatment after complete tumor resection.
Figure 1: Primary endpoint of the ADAURA study: disease-free survival in patients with stage II/IIIA disease
CTONG1104: OS for gefitinib vs. chemotherapy
The randomized, phase III CTONG 1104 trial has established a significant DFS benefit of adjuvant treatment with the first-generation EGFR TKI gefitinib 250 mg daily compared to standard doublet chemotherapy consisting of vinorelbine plus cisplatin in EGFR-mutant, completely resected stage II/IIIA NSCLC [8]. Wu et al. presented the final OS results after a median follow-up of 80.0 months at the ASCO Congress [9]. The intent-to-treat (ITT) population included 111 patients in each treatment arm, while the per-protocol (PP) population included 106 and 87 patients in the gefitinib and chemotherapy arms, respectively.
According to the analysis, gefitinib gave rise to survival benefits compared to chemotherapy with median OS of 75.5 vs. 62.8 months in both populations, although this difference was not significant (HR, 0.92). Five-year OS rates were 53.2 % vs. 51.2 % in the ITT group, with similar results for the PP population. The authors noted that the OS finding for the gefitinib arm was among the best observed in completely resected IIB/IIIA NSCLC compared to historical data [10]. Updated findings for DFS showed a significant benefit for gefitinib (30.8 vs. 19.8 months), with reductions in the risk of recurrence and death of 44 % and 49 % in the ITT and PP populations, respectively (p = 0.001 and < 0.001, respectively). However, this advantage did not translate into a significant OS difference.
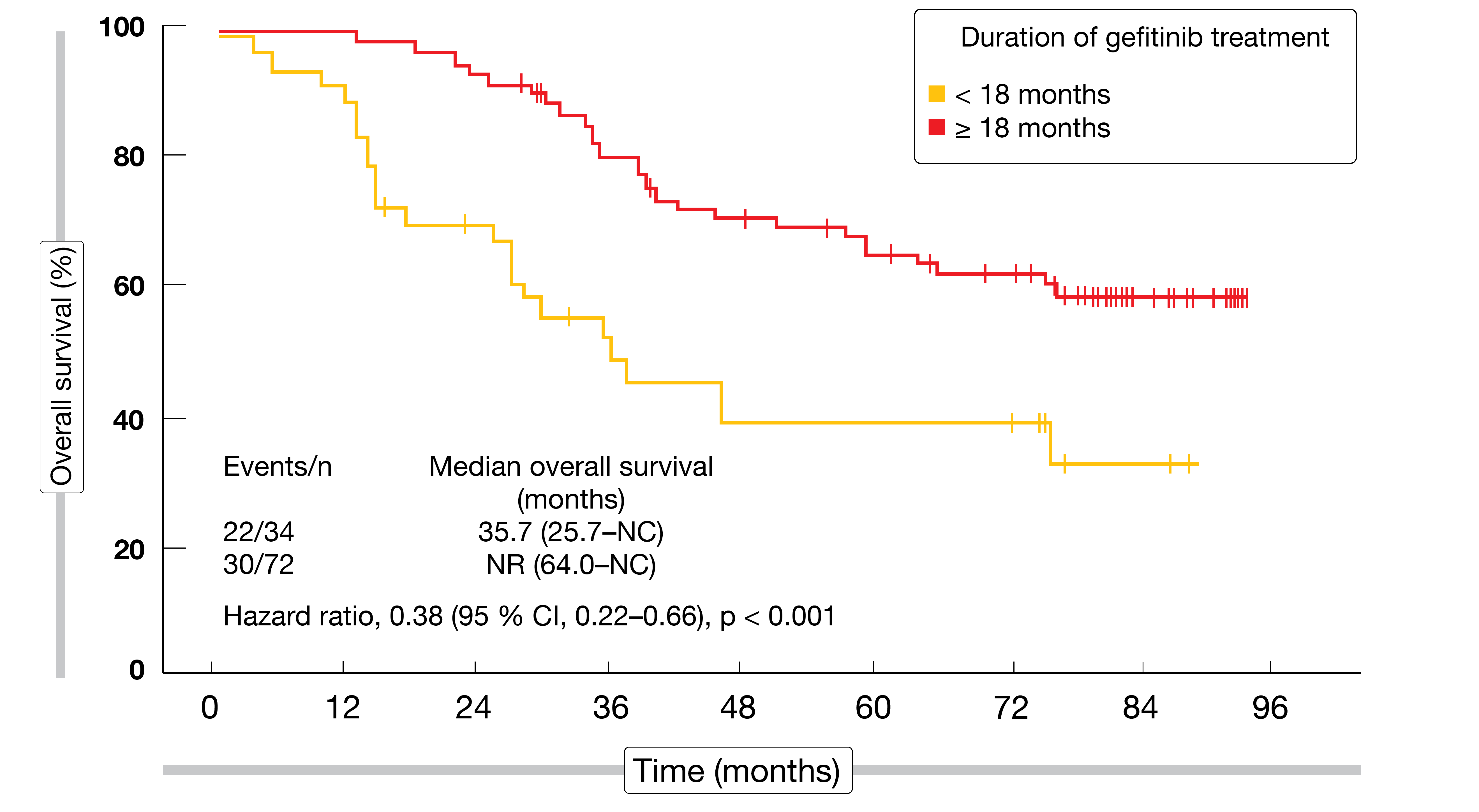
According to a post-hoc analysis, the patients in the gefitinib arm who received subsequent TKI treatment achieved the longest OS compared to patients with other or no subsequent therapies across the two arms (p < 0.001). At 55.6 %, the response rate was highest in individuals treated with gefitinib followed by osimertinib. Moreover, patients receiving gefitinib for at least 18 months experienced significantly better OS than those with a treatment duration < 18 months (HR, 0.38; p < 0.001; Figure 2). The authors concluded that adjuvant EGFR TKI therapy should be considered as the optimal modality to improve DFS and achieve potentially prolonged OS in patients with completely resected, EGFR-mutated, stage II/IIIA NSCLC.
Figure 2: Overall survival in relation to the duration of adjuvant gefitinib therapy in the CTONG1104 trial
Atezolizumab prior to chemoradiation
Neoadjuvant PD-1/PD-L1 blockade in early-stage NSCLC has been shown to be feasible and associated with high pathological response rates [11-14]. Therefore, the single-arm, phase II AFT-16 trial explored the risks and benefits of neoadjuvant atezolizumab before definitive chemoradiation (CRT) in patients with unresectable stage IIIA/B NSCLC [15]. Sixty-two individuals were included in the analysis. They received a total of four cycles of atezolizumab 1,200 mg three-weekly before CRT. After CRT, adjuvant atezolizumab was administered to complete one year of treatment.
The disease control rate at 12 weeks, which constituted the primary endpoint, was 77.4 %. Baseline PD-L1 expression status was available for 49 patients. In the groups with PD-L1 expression < 1 % and ≥ 1 %, disease control resulted in 82.4 % and 90.9 %, respectively. Atezolizumab prior to and following CRT was well tolerated, with AEs mostly reported as grade-1 events. AEs of special interest included hyperthyroidism, hypothyroidism, rash, anaphylactic reaction, colitis and Guillain-Barré syndrome in a total of 10 patients. As the authors noted, further study of induction immune checkpoint inhibitor therapy is warranted in the setting of unresectable stage III NSCLC.
SABR in conjunction with atezolizumab
Stereotactic ablative radiotherapy (SABR) is used in inoperable, early-stage NSCLC, although regional and distant failures remain an issue [16]. Data have demonstrated synergy between radiation and immune checkpoint inhibition, suggesting that neoadjuvant delivery of checkpoint blockade might be superior to adjuvant-only delivery [17, 18]. Based on these observations, Kelly et al. conducted a phase I study to assess the safety and maximum tolerated dose of neoadjuvant, concurrent, and adjuvant atezolizumab with SABR in high-risk, early-stage NSCLC [19]. Patients with inoperable NSCLC (T1-3 N0 M0) and at least one feature predictive of high recurrence risk, such as certain tumor diameters or poorly differentiated histology, received six cycles of atezolizumab at three dose levels, while five fractions of SABR at doses of 10 Gy to 12.5 Gy per fraction were delivered concurrently during cycle 3. Fifteen out of 20 patients completed all six cycles.
Atezolizumab 1,200 mg/kg was identified as the recommended phase II dose. Re-staging after the initial two cycles of atezolizumab already showed signs of anti-tumor activity, with unconfirmed partial remissions in 22 %. Median PFS in the total cohort of 20 patients was 25.5 months. In those with PD-L1–positive tumors, PFS was almost double that observed in the PD-L1–negative group (30.0 and 16.3 months, respectively). Overall, atezolizumab administered before, during and after SABR proved feasible and well tolerable. Treatment-related AEs, such as cytopenia, fatigue, rash and diarrhea were mainly limited to grade 1 and 2 events. Two cases of pneumonitis were graded as 1 and 2. Additional blood and tissue biomarker analyses are ongoing, as an inflamed tumor microenvironment might be associated with response. Moreover, the combination of atezolizumab and SABR is presently being tested in the randomized phase III SWOG/NRG S1914 trial.
Perioperative durvalumab
Neoadjuvant chemotherapy with three cycles of cisplatin and docetaxel followed by two cycles of durvalumab 750 mg/m² two-weekly prior to surgery was investigated by the multicenter, single-arm, phase II SAKK 16/14 trial [20]. After surgery, durvalumab treatment continued for one year. Sixty-seven patients with resectable stage IIIA NSCLC (T1-3 N2 M0) were included in the study. Event-free survival (EFS) at 12 months was defined as the primary endpoint.
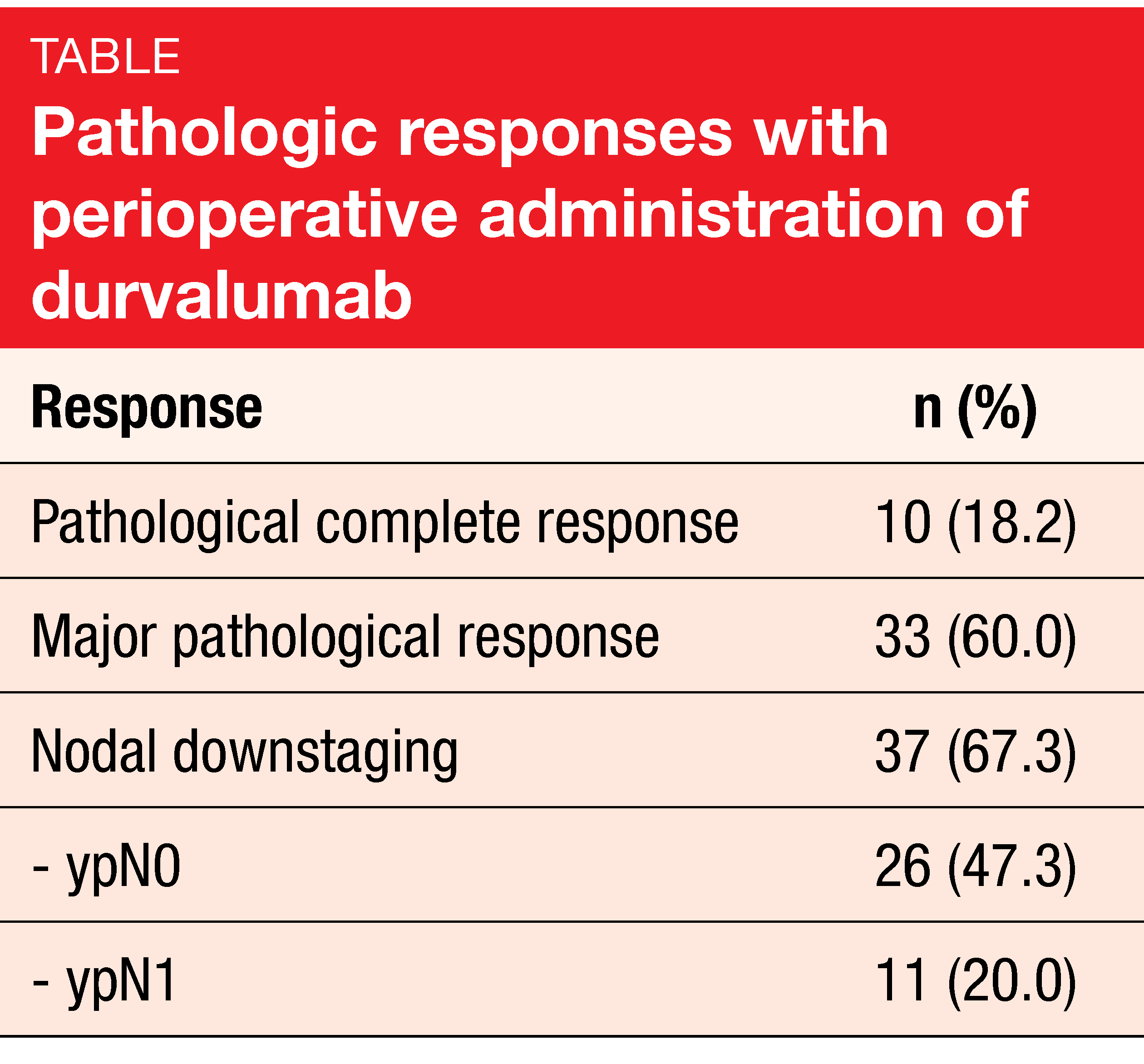
Fifty-eight patients completed neoadjuvant immunotherapy, and 55 underwent surgery. In 91 % of cases, R0 resection was achieved. Pathologic complete responses resulted in 18.2 % of patients, and nodal downstaging was obtained in 67.3 % (Table). Also, the analysis revealed a high rate of major pathological responses. EFS at 12 months amounted to 73.3 %, with median EFS not having been reached at the time of the analysis. Likewise, median OS had not been reached yet. The 30-day postoperative mortality rate was 1.8 %.
According to the authors, the addition of perioperative durvalumab to standard-of-care cisplatin/docetaxel is safe and resulted in an encouraging 1-year EFS rate, which exceeded historical data of chemotherapy alone. Perioperative PD-L1 inhibition in addition to standard neoadjuvant chemotherapy forms the backbone of the SAKK 16/18 study that will evaluate the benefit of neoadjuvant immunomodulatory radiotherapy.
Induction chemotherapy plus radiation or bevacizumab
The randomized phase II PIT-1 study assessed platinum doublet chemotherapy plus concurrent thoracic radiation therapy (TRT) or bevacizumab followed by surgery in 88 patients with stage IIIA (N2) non-squamous NSCLC [21]. In the TRT and bevacizumab arms, 37 and 38 patients, respectively, underwent surgery. R0 resection was possible in 97 % and 89 %, respectively.
Regarding the 2-year PFS rate, which constituted the primary endpoint, the analysis demonstrated a 50 % benefit with the TRT regimen that was superior to the rate of 36.8 % obtained in the bevacizumab arm. Also, major pathological responses occurred more frequently in the TRT group (49 % vs. 14 %). Two-year OS rates were 80% in both arms. Most of the treatment-related AEs were well balanced, although grade 1-3 hypertension occurred more often with bevacizumab, while grade 1/2 esophagitis and dermatitis were restricted to the TRT-based regimen. Fatal surgical complications due to bronchopleural fistula were only observed in the bevacizumab group (two cases). Based on these findings, the authors chose pemetrexed/cisplatin plus concurrent TRT as the investigational induction regimen for a future phase III study.
REFERENCES
- Datta D, Lahiri B, Preoperative evaluation of patients undergoing lung resection surgery. Chest 2003; 123(6): 2096-2103
- Le Chevalier T, Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann Oncol 2010; 21 Suppl 7: vii196-vii198
- Cagle PT et al., Lung cancer biomarkers: Present status and future developments. Arch Pathol Lab Med 2013; 137(9): 1191-1198
- Kris MG et al., Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline update. J Clin Oncol 2017; 35(25): 2960-2974
- Pignon JP et al., Lung Adjuvant Cisplatin Evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008; 26(21): 3552-3559
- Soria JC et al., Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378(2): 113-125
- Herbst RS et al., Osimertinib as adjuvant therapy in patients with stage IB-IIIA EGFR mutation positive NSCLC after complete tumor resection: ADAURA. J Clin Oncol 38: 2020 (suppl; abstr LBA5)
- Zhong WZ et al., Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open-label, phase 3 study. Lancet Oncol 2018; 19(1): 139-148
- Wu YL et al., CTONG1104: Adjuvant gefitinib versus chemotherapy for resected N1-N2 NSCLC with EGFR mutation – final overall survival analysis of the randomized phase 3 trial. J Clin Oncol 38: 2020 (suppl; abstr 9005
- Goldstraw P et al., The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11(1): 39-51
- Forde PM et al., Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018; 378(21): 1976-1986
- Kwiatkowski DJ et al., Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 37, 2019 (suppl; abstr 8503
- Cascone T et al., Neoadjuvant nivolumab or nivolumab plus ipilimumab for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J Clin Oncol 37, 2019 (suppl; abstr 8504
- Provencio M et al., NEO-adjuvant chemo-immunotherapy for the treatment of stage IIIA resectable non-small-cell lung cancer (NSCLC): A phase II multicenter exploratory study – final data of patients who underwent surgical assessment. J Clin Oncol 37, 2019 (suppl; abstr 8509
- Ross HJ et al., AFT-16: Phase II trial of atezolizumab before and after definitive chemoradiation (CRT) for unresectable stage III non-small cell lung cancer (NSCLC). J Clin Oncol 38: 2020 (suppl; abstr 9045)
- Timmerman R et al., Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303(11): 1070-1076
- Young KH et al., Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One 2016; 11(6): e0157164
- Dovedi SJ et al., Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014; (74)19: 5458-5468
- Kelly K et al., Atezolizumab plus stereotactic ablative therapy for medically inoperable patients with early-stage non-small cell lung cancer. J Clin Oncol 38: 2020 (suppl; abstr 9011
- Rothschild SI et al., SAKK 16/14: Anti-PD-L1 antibody durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small cell lung cancer (NSCLC) – a multicenter single-arm phase II trial. J Clin Oncol 38: 2020 (suppl; abstr 9016)
- Takamochi K et al., PIT-1: Randomized phase II trial of pemetrexed-cisplatin plus bevacizumab or concurrent thoracic radiation therapy followed by surgery in stage IIIA (N2) nonsquamous non-small cell lung cancer. J Clin Oncol 38: 2020 (suppl; abstr 9014)
More posts
Rare mutations: HER2, RET, ALK, BRAF
Trastuzumab deruxtecan (T-DXd) is a novel antibody-drug conjugate containing a humanized anti-HER2 monoclonal antibody linked to a topoisomerase I inhibitor exatecan derivative. The open-label, multicenter, phase II DESTINY-Lung01 study tested T-DXd 6.4 mg/kg 3-weekly in patients with relapsed or refractory advanced NSCLC that expressed HER2 (Cohort 1; n = 42) or carried HER2-activating mutations (Cohort 2; n = 42).
COVID-19 in patients with thoracic cancers: TERAVOLT
The global consortium TERAVOLT was established to determine factors that place patients with thoracic malignancies who develop COVID-19 at risk for hospitalization and death, to elucidate the clinical course of these patients and to identify therapeutic strategies that might impact survival. Thoracic cancer patients with a COVID-19 diagnosis, i.e. cases of confirmed infection according to RT-PCR techniques and suspected COVID-19 cases, are being entered into the database.
Present and future perspectives of anti-angiogenic therapy
The oral, triple angiokinase inhibitor nintedanib has been approved in the European Union and other countries in combination with docetaxel for the treatment of advanced adenocarcinoma of the lung after first-line chemotherapy. It works by targeting vascular endothelial growth factor (VEGF) receptors 1-3, platelet-derived growth factor (PDGF) receptors α/β and fibroblast growth factor (FGF) receptors 1-3, as well as RET.
Improving outcomes in the early-stage setting with (neo)adjuvant strategies
Approximately 30 % of NSCLC patients present with resectable disease at diagnosis. Surgery is the primary treatment for early-stage NSCLC; after resection, adjuvant cisplatin-based chemotherapy is recommended for patients with stage II/IIIA lung cancer and select patients with stage IB disease. However, the rates for disease recurrence or death following surgery and adjuvant chemotherapy remain high, ranging from 45 % in stage IB to 76 % in stage III.
EGFR-mutated disease: early combinations and new approaches in exon 20 insertion-positive lung cancer
Oligometastatic disease is generally defined by one to five metastatic lesions. As progression occurs most frequently in sites of the original disease, it is surmised that aggressive local treatment might prevent further dissemination. Based on this rationale, the open-label, randomized, phase III SINDAS trial conducted in China explored the use of concurrent stereotactic body radiotherapy (SBRT) and EGFR TKI therapy in patients with oligometastatic, EGFR-mutant NSCLC.
Immune checkpoint inhibition: comprehensive benefits, but not devoid of risks
First-line nivolumab plus ipilimumab (NI) was shown to significantly prolong OS compared to chemotherapy in patients with advanced NSCLC irrespective of tumor PD-L1 expression in the randomized, phase III CheckMate 227 study. At the ASCO Congress, Ramalingam et al. presented the updated 3-year efficacy and safety results from Part 1 of the trial.