Malignant mesothelioma: recent data on nintedanib and checkpoint inhibitors
Malignant pleural mesothelioma (MPM) is an aggressive tumour that, if left untreated, shows a median survival of 7–9 months [1]. The front-line standard treatment for patients with unresectable MPM consists of combination doublet therapy with cisplatin and pemetrexed, which yields a median OS of approximately 1 year.
Biomarker analysis of the LUME-Meso trial
The angiokinase inhibitor nintedanib has been tested successfully in MPM patients in the randomised, double blind, placebo-controlled phase II/III LUME-Meso trial. In the phase II part of the study, 87 chemotherapy-naïve patients with unresectable epithelioid or biphasic MPM received either nintedanib plus pemetrexed/ cisplatin or placebo plus chemotherapy. Here, the addition of nintedanib to chemotherapy improved PFS to a clinically meaningful extent (9.4 vs. 5.7 months; HR, 0.54; p = 0.010), and there was a trend towards improvement in OS (18.3 vs. 14.2 months; HR, 0.77; p = 0.319) [2]. The efficacy of treatment was most pronounced in patients with epithelioid histology.
Nowak et al. conducted an exploratory biomarker analysis in the epithelioid population that covered plasma levels of 58 angiogenic factors, SNPs in genes for mesothelin, VEGFR1 and VEGFR3, and microvessel density [3]. Predictive and prognostic analyses were performed for OS and PFS. However, none of these biomarkers showed a clear association with treatment benefit after false discovery rate adjustment, although the analyses were limited by the small sample size. The only marker that was predictive for both OS and PFS was plasma endoglin, with higher levels suggesting smaller benefits from the addition of nintedanib. VEGF-D appeared to have a certain predictive value for OS, but this did not hold true for PFS. For SNPs, there was a signal that two VEGFR3 polymorphisms might be predictive of lesser benefit of nintedanib. These findings will be evaluated further in the phase III part of the study.
The confirmatory phase III part of the LUME-Meso trial is currently enrolling patients with unresected epithelioid MPM at approximately 140 centres worldwide [4]. Nintedanib plus pemetrexed/ cisplatin followed by nintedanib maintenance is being compared with placebo plus chemotherapy followed by placebo maintenance. PFS constitutes the primary endpoint.
Immunotherapeutic approaches
Goto et al. assessed the use of the checkpoint inhibitor nivolumab in the second-line or third-line setting [5]. Thirty-four patients with advanced or metastatic MPM who were resistant or intolerant to platinum-based combination therapy with pemetrexed participated in the MERIT trial. ORR was 29.4 % in the total population. Patients with all histologies responded to treatment: ORRs for epithelioid, sarcomatoid and biphasic histology were 25.9 %, 66.7 %, and 25.0 %, respectively. The DCR amounted to 67.6 %. Median PFS was 6.1 months; at 6 months, half of the patients remained progression-free. Median OS had not been reached at the time of the analysis, with a 6-month OS rate of 85.3 %. The toxicity profile proved manageable. Grade 3/4 AEs occurred in 11.8 % of patients, and AEs necessitated treatment discontinuation in 5.9 %.
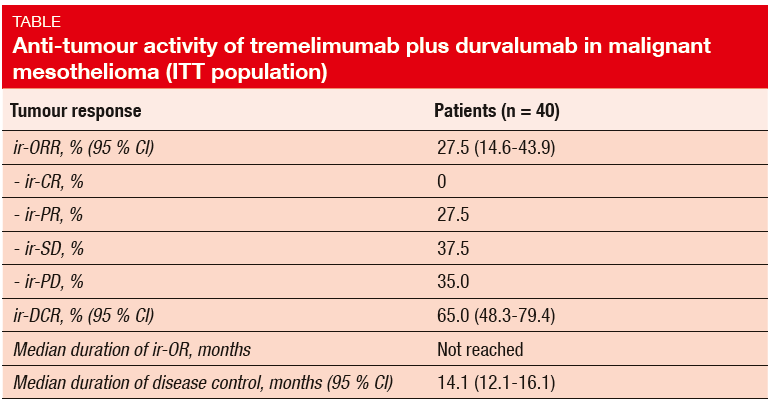
The NIBIT-MESO-1 trial investigated the combination of the anti-CTLA-4 antibody tremelimumab with the anti-PD-L1 antibody durvalumab in 40 mesothelioma patients who were refractory to or had relapsed after first-line chemotherapy, or had refused it [6]. The study met its primary objective, which was defined as immunerelated (ir) ORR. In the ITT population, ir-ORR was 27.5 %, and an additional 37.5 % of patients achieved ir-SD (Table). This translated into an ir-DCR of 65.0 %. Median duration of ir-OR had not been reached at the time of the analysis, and median duration of disease control was 14.1 months.
Immune-related AEs of any grade occurred in 75 % of patients; grade 3/4 AEs were observed in 17.5 %. Treatment-related AEs were generally manageable and reversible. The authors concluded that the combination of tremelimumab and durvalumab is active and shows a good safety profile in malignant mesothelioma. Further ex-ploration is warranted.
REFERENCES
- Hiddinga BI et al., Mesothelioma treatment: Are we on target? A review. J Adv Res 2015; 6: 319-330
- Nowak AK et al., Mature overall survival (OS) results from the LUME-Meso study of nintedanib (N) + pemetrexed/cisplatin (PEM/CIS) vs placebo (P) + PEM/CIS in chemo-naïve patients (pts) with malignant pleural mesothelioma (MPM). J Clin Oncol 35, 2017 (suppl; abstr 8506)
- Nowak AK et al., Nintedanib + pemetrexed/cisplatin in malignant pleural mesothelioma: phase II biomarker data from the LUME-Meso study. WCLC 2017, MA 19.03
- Tsao AS et al., LUME-Meso phase II/III study: nintedanib + pemetrexed/cisplatin in chemotherapy-naïve patients with malignant pleural mesothelioma. WCLC 2017, P1.09-011
- Goto Y et al., A phase II study of nivolumab: a multicenter, open-label, single arm study in malignant pleural mesothelioma (MPM); MERIT. WCLC 2017, MA 19.01
- Calabrò L et al., Tremelimumab plus durvalumab in first- or second-line mesothelioma patients: final analysis of the NIBIT-MESO-1 study. WCLC 2017, MA 19.02