Novel approaches in small-cell lung cancer
Small-cell lung cancer (SCLC) accounts for approximately 15 % of all lung cancers, and it is associated with poor outcomes [1]. 70 % of these patients present with extensive disease. Their treatment remains a significant challenge for oncologists. The median survival in the extensive disease stage is 7 months to 9 months, and only 2 % of patients survive for 5 years [2]. So far, many attempts to improve upon these results in the first-line setting have failed.
Patients with SCLC usually respond to initial platinum-based chemotherapy, but the response is rapidly followed by progression. After that, treatment options become limited. At the ASCO Congress, the results obtained with two new chemotherapy approaches were presented, as well as data on two trials that explored immunotherapeutic agents in relapsed disease.
Toxicity-adjusted chemotherapy dosing: STAD-1
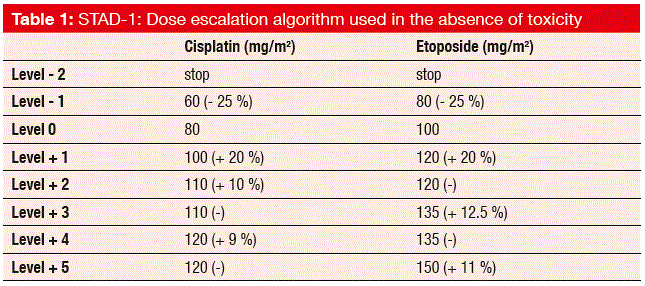
The STAD-1 trial attempted to optimise chemotherapy by comparing toxicity-adjusted dosing of cisplatin and etoposide with fixed dosing of the same drugs in patients with advanced SCLC [3]. This concept is based on the observation that haematological toxicity due to cytotoxic agents might serve as a surrogate biomarker of drug activity. Between 2008 and 2014, 11 Italian centres participated in this trial. A total of 161 chemotherapy-naïve patients were randomly assigned to either fixed-dose cisplatin 80 mg/m2 on day 1 plus etoposide 100 mg/m2 on days 1 to 3, every 3 weeks (n = 81), or to dose adjustment of both cisplatin and etoposide within a range of eight dosing levels (level -2 to level +5; Table 1) according to the toxicity after the start of the treatments (n = 80). If grade 2 to grade 4 neutropenia or unacceptable toxicity were not present in a given cycle, the dose level had to be escalated for the next cycle. De-escalation occurred for unacceptable toxicity. The ORR was defined as the primary endpoint of the trial.
As expected, the side effects increased considerably in the experimental arm, where there was a predominance of severe AEs (grade ≥ 3) at 30 % over the control arm (90 % vs. 60 %; p < 0.0001). Six deaths occurred during treatment, one in the control arm (for unknown reasons), and five in the experimental arm (3 infections, 1 febrile neutropenia, 1 vascular death). However, no differences between the two approaches were observed regarding the ORR (57 % vs. 54 %, for the toxicity-adjusted and fixed-dose therapies, respectively), PFS (5.6 vs. 6.0 months) and OS (9.2 vs. 9.6 months). There was a higher range of doses in the experimental group, but the median dose intensities did not differ between these treatment arms.
MATISSE: palifosfamide as part of the team
Another strategy that was aimed at improving standard platinum chemotherapy for extensive-stage SCLC was tested in the MATISSE trial [4]. A prior phase III study demonstrated improved survival with etoposide plus cisplatin and ifosfamide over etoposide plus cisplatin alone [5]. However, increased toxicity occurred. Also, the administration of ifosfamide requires hospitalisation and the administration of mesna for the prevention of drug-induced haemorrhagic cystitis. Therefore, research efforts focused on the identification of an equally effective but less toxic third drug.
The multi-centre, open-label, MATISSE study tested the addition of palifosfamide 130 mg/m2 on days 1 to 3 to etoposide 100 mg/m2 on days 1 to 3 and cisplatin AUC 4 on day 1 as compared to etoposide 100 mg/m2 on days 1 to 3 plus cisplatin AUC 5 on day 1, for 4 to 6 cycles. Overall, 118 chemotherapy-naïve patients participated in the study, with OS defined as the primary outcome.
The addition of palifosfamide indeed added little toxicity to this comparator regimen, but it did not give rise to any improvement in the OS either. On the contrary, survival was numerically inferior in the experimental arm (10.0 vs. 10.4 months). MATISSE was closed prematurely, and palifosfamide is no longer being developed for the treatment of SCLC.
Preliminary results on pembrolizumab: KEYNOTE-028
Pembrolizumab, an anti-PD-1 monoclonal antibody, has shown antitumour activity in multiple advanced malignancies. The KEYNOTE programme is investigating pembrolizumab in a variety of tumour types. Patients with SCLC have been included in the ongoing KEYNOTE-028 phase Ib multicohort study that is assessing pembrolizumab monotherapy in PD-L1–positive solid tumours [6]. In the SCLC cohort, 20 patients who were not able to receive standard therapy or who had experienced treatment failure were treated with pembrolizumab 10 mg/kg IV every 2 weeks. If complete or partial responses or stable disease was achieved, the therapy was continued for 24 months or until progression.
Pembrolizumab was shown to be generally well tolerated and to have promising antitumour activity. The overall response rate was 35 %, and stable disease occurred in 5 % of these patients. At the time of the data cut-off, six of seven responses were ongoing. However, as the authors pointed out, these results are still early. The safety and toxicity profiles are consistent with previous observations for pembrolizumab in patients with other tumour types.
CheckMate 032: concurrent nivolumab and ipilimumab
Combined block of the PD-1 and CTLA-4 immune checkpoint pathways shows solid antitumour activity, with a manageable safety profile. The open-label, randomised, CheckMate 032 phase I/II study investigated the fully human IgG4 PD-1 immune checkpoint inhibitor nivolumab with or without the CTLA-4 checkpoint inhibitor ipilimumab for the treatment of recurrent SCLC [7].
Platinum-sensitive and platinum-refractory patients with progressive disease after at least one prior line of therapy (including a platinum-based regimen in the first line) participated regardless of their tumour PD-L1 status or number of prior chemotherapy regimens. They were randomised to either nivolumab monotherapy at a dose of 3 mg/kg every 2 weeks or to the combination of nivolumab and ipilimumab IV. While 40 patients received nivolumab alone, 50 were treated with nivolumab 1 mg/kg plus ipilimumab 1 mg/kg IV or nivolumab 1 mg/kg plus ipiliumumab 3 mg/kg IV, every 3 weeks, for 4 cycles. After these 4 cycles of the combination, all of the patients received nivolumab 3 mg/kg every 2 weeks, as maintenance therapy.
Approximately half of the patients in the combination arm and one third of those in the monotherapy arm were treated in the second-line setting. The other trial participants were treated in the third line and beyond. One third of the entire cohort were platinum-refractory. At the time of assessment of clinical activity, the experimental arm consisted of 46 patients, as four had not reached the first tumour assessment at the database lock.
Immunotherapy shows promise
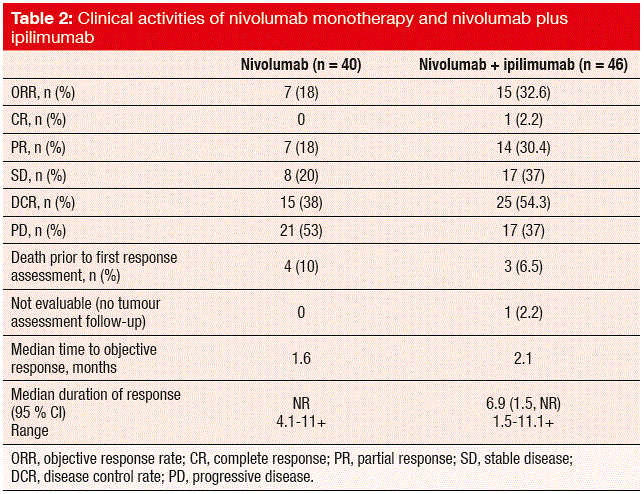
As the primary objective, the ORR was higher for the combination than for nivolumab monotherapy (32.6 % vs. 17.4 %; Table 2). Partial responses occurred in 30.4 % vs. 18.0 % of the patients in the experimental and control arms, respectively. Importantly, durable responses were observed. Overall, DCR achieved with nivolumab plus ipilimumab was superior to nivolumab monotherapy (54.4 % vs. 38 %). The median OS was 8.2 months and 4.4 months, respectively. A subset analysis of the second-line patients demonstrated that even patients with platinum-refractory disease experienced profound responses to both of these therapies. The onset of responses was generally rapid. Occasional pseudo-progression was followed by tumour regression. PD-L1 expression did not appear to be predictive for the responses, as many PD-L1-negative patients responded well to both combination therapy and monotherapy.
The safety profiles of both of these treatment regimens were consistent with those seen in other tumour types, and these were managed according to the established safety guidelines. Treatment-related AEs occurred more frequently in the combination arm (77 % vs. 53 %). This was also true for grade 3/4 AEs (34 % vs. 15 %). Of note, diarrhoea, rash, hypothyroidism, hyperthyroidism, and lipase elevations were seen mainly in the experimental arm. There were no grade 3/4 cases of pneumonitis in the nivolumab arm, while one patient was affected in the combination cohort. Development of autoimmune disease (myasthenia gravis) or paraneoplastic syndromes is a possible issue, however. Three patients developed limbic encephalitis, which was resolved under immunosuppressive treatment in two cases.
Based on these encouraging results, nivolumab monotherapy and the combination therapy with ipilimumab will be explored in future SCLC trials.
REFERENCES
- National Cancer Institute PDQ®. Small Cell Lung Cancer. http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional/page1
- Jackman DM & Johnson BE, Small-cell lung cancer. Lancet 2005; 366(9494): 1385-96
- Morabito A et al., A multicenter, randomised, phase III trial comparing fixed dose versus toxicity-adjusted dose of cisplatin + etoposide in advanced SCLC patients. The STAD-1 trial. J Clin Oncol 33, 2015 (suppl; abstr 7505)
- Jalal SI et al., Results from a randomized study of carboplatin and etoposide (CE) with or without palifosfamide (Pa) in extensive stage small cell lung cancer (ES-SCLC): The MATISSE study. J Clin Oncol 33, 2015 (suppl; abstr 7504)
- Loehrer PJ Sr et al., Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol 1995; 13(10): 2594-9
- Ott PA et al., Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol 33, 2015 (suppl; abstr 7502)
- Antonia SJ et al., Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Clin Oncol 33, 2015 (suppl; abstr 7503)