PD-L1 expression is a nightmare in terms of complexity
Martin Reck, MD, PhD, Department of Thoracic Oncology, Lung Clinic Grosshansdorf, Germany
Immunotherapies offer advantages in unselected patients. However, the implementation of biomarkers would be highly welcome. One of the reasons for this would be containment of the financial strain on health systems, as physicians could then exclude patients who are unlikely to benefit from treatment. Where are we today regarding biomarker development?
There are two issues that are tied to the topic of biomarkers in immunotherapies. First, all attempts to define biomarkers have focused primarily on response as a marker of efficacy. We have to be aware, however, that immunotherapies are not targeted therapies, and fast tumor shrinkage is not necessarily observed with this kind of treatment. The efficacy of an immunotherapy is defined by long-lasting tumor stabilization. We have to question whether response rates are the appropriate endpoint here.
The second issue is the necessity to define the purpose of biomarker development in immunotherapies. There are two options: Biomarkers can identify patients who derive benefit from a specific treatment, or they can exclude those patients who are unlikely to benefit. This is something that needs to be defined. Currently, both strategies are being explored.
What kind of evidence is available at present?
Based on analyses from the CheckMate trials, it can be said that neither patient age nor performance status have any impact on the efficacy of anti-PD-1 antibodies. The same is true for tumor histology. We have seen long-lasting responses to anti-PD-1 antibodies and anti-PD-L1 antibodies in patients with both squamous and non-squamous tumors. One factor of interest is smoking. The CheckMate trials have revealed superior response rates in the group of smokers or former smokers [1, 2]. This signal was found with other anti-PD-1/PD-L1 agents as well; for pembrolizumab, increased benefit was also apparent in terms of PFS and OS [3]. Smoking is known to induce a chronic inflammatory response in pulmonary tissue. Moreover, lung cancers of smokers have 10 times as many mutations as those of non-smokers. A high mutational load contributes to the immunogenicity of a tumor and might be correlated to the efficacy of immunotherapies. Data from Rizvi demonstrated an interesting association between the mutational load and the efficacy of pembrolizumab treatment in patients with pre-treated NSCLC [4]. This applied to response rates and PFS. A molecular smoking signature was developed that correlated with these endpoints. Perhaps an inflammatory signature will be relevant in the future. Interesting data obtained with the anti-PD-L1 antibody durvalumab have suggested that there is a group of tumors that is driven by inflammation and that is highly susceptible to immunotherapy [5]. Disappointingly, no blood-based markers with any predictive power for the use of anti-PD-1/PD-L1 antibodies have been identified so far.
What do we know about tumors with low mutational burden; for example, those with activating EGFR mutations?
These data are limited and explorative. However, some signals were derived from the CheckMate 057 trial. It appears that the efficacy of nivolumab compared to docetaxel is slightly inferior in the group of patients with positive EGFR mutation status [6, 7]. Similar signals were observed for other anti-PD-1/PD-L1 antibodies, like atezolizumab [8, 9] and pembrolizumab [10, 11].
PD-L1 expression has been the main focus of biomarker research for quite some time. How would you rate the relevance of this marker?
PD-L1 expression is independent of any other molecular marker. A clear correlation across all anti-PD-1/PD-L1 antibodies has been found between PD-L1 expression status and response, as well as PFS and OS, even in the first-line setting. However, we must be aware that PD-L1–negative patients can have 1-year OS rates of 70 %.
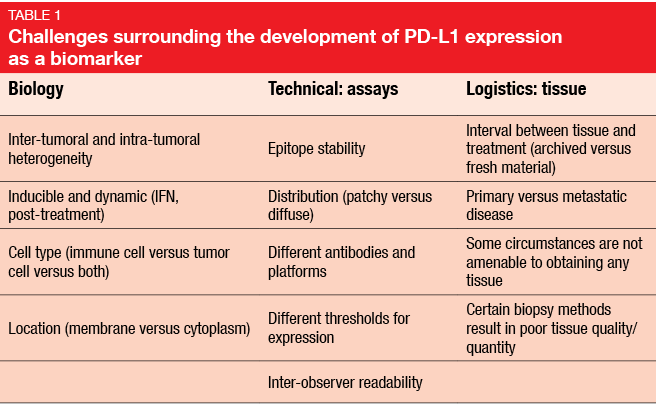
PD-L1 is not an easy-to-handle marker. Actually, it is a nightmare in terms of complexity, with a number of issues regarding biology, assays, and logistics (Table). In practice, different assays are used for measurement, and with different definitions of PD-L1 positivity added in, the story of PD-L1 assessment becomes very complicated. At the moment, at least four different test methods are in use. This will not be practical if we are going to apply immunotherapy in the clinic.
Undoubtedly, further development and harmonisation of PD-L1 assessment is urgently needed. An ongoing global initiative, which was put together by drug manufacturers, companies that provide testing systems, scientific societies, and regulatory authorities, is aimed at providing practical guidance on the use of PD-L1 expression.
What about additional markers?
For PD-L2, high concordance with PD-L1 expression has been demonstrated, and there is an association with the response to anti-PD-1 antibodies after adjustment for PD-L1 expression [12]. PD-L2 is highly expressed in tumors, in the endothelial and stromal cells of NSCLC, but minimally expressed in normal tissue. Furthermore, infiltration of CD8+ cells into the ‘invasive margin’ of tumors is of interest. Tumor response to anti-PD-1/PD-L1 antibodies was shown to correlate with the density of pre-existing D8+ cells [13].
Apart from EGFR mutation, which molecular targets will gain importance in the future?
Data are coming up with respect to new targeted therapies; for example, for BRAF inhibitors. BRAF mutation occurs in 2 % to 3 % of our lung-cancer patients. We have already seen very interesting data in this area. A trial on the combination of a MEK inhibitor and a BRAF inhibitor in patients with BRAF mutation is ongoing. I believe that a targeted option will become available for this group of patients. Another druggable target is RET translocation. To my mind, the future will not be driven primarily by identification of molecular targets, but rather by identification of resistance mechanisms after first-line targeted therapies, and the development of adequate treatments in the refractory third-line situation.
We are at a stage now where we can talk about the whole treatment. The patients will not be cured, but we can offer them a long period of disease-free survival or stable disease. The sequence of therapies will become very important, which also applies to the possibility of offering the patient another active compound after progression. This is already a reality in EGFR-mutated patients; after progression, treatment can be continued with a third-generation agent. In the future, fourth-generation or fifth-generation TKIs might become available.
What are the greatest challenges at present in the treatment of lung-cancer patients from a practical point of view?
One of the greatest challenges is the dynamics of drug development. A great number of new compounds is available, and the treatment has become very complex. The greatest challenge, however, is the accessibility of the tumor tissue. In lung-cancer patients, it is sometimes extremely difficult to procure sufficient material for all of the required molecular or translational analyses. The question of whether we can develop molecular tests that work with a limited amount of tissue will soon be of paramount importance. Another challenge, of course, is the pharmaco-economic impact of the new drugs. Immunotherapeutics are extremely expensive. It remains to be seen whether the health care systems can provide access to these compounds for all patients.
REFERENCES
- Reckamp K et al., WCLC 2015, abstract 736
- Paz-Ares L et al., Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small-cell lung cancer (NSCLC). J Clin Oncol 2015; 33(suppl; abstr LBA109)
- Hellmann MD, WCLC 2015
- Rizvi NA et al., Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small-cell lung cancer. Science 2015; 348(6230): 124-128
- Higgs B et al., High tumoral IFNγ mRNA, PD-L1 protein, and combined IFNγ mRNA/PD-L1 protein expression associates with response to durvalumab (anti-PD-L1) monotherapy in NSCLC patients. Ann Oncol 2015; 26(suppl 6): abstract 15LBA
- Gettinger S et al., CMSTO 2014
- Hellmann MD, ESMO 2014, abstract 6111
- Horn L et al., WCLC 2013, abstract 2347
- Soria J et al., Clinical activity, safety and biomarkers of PD-L1 blockade in non-small-cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PD-L1). ECC 2013, abstract 3408
- Hellmann et al., Efficacy of pembrolizumab in key subgroups of patients with advanced NSCLC. J Thorac Oncol 2015; 10(suppl 2): MINI03.05
- Garon E et al., Antitumor activity of pembrolizumab (Pembro; MK-3475) and correlation with programmed death ligand 1 (PD-L1) expression in a pooled analysis of patients (pts) with advanced non-small-cell lung carcinoma (NSCLC). ESMO 2014, abstract LBA43
- Yearley J et al., PD-L1 expression in human tumors: relevance to anti-PD-1 therapy in cancer. ECC 2015, abstract 18LBA
- Tumeh PC et al., D-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515(7528): 568-571