PFS improvement due to local therapy in oligometastatic NSCLC
Evidence suggests the existence of a ,limited metastatic’ NSCLC phenotype. However, the type of optimal treatment and the role of aggressive local therapy in these patients remain controversial. Gomez et al. presented the first prospective, randomised trial to address this question. Patients had stage IV disease without RECIST progression and a maximum of three metastases after front-line systemic therapy (FLST). Malignant pleural effusion was an exclusion criterion. FLST was defined as ≥ 4 cycles of platinum-doublet chemotherapy, ≥ 3 months of erlotinib, afatinib, or gefitinib therapy in case of EGFR mutation, or ≥ 3 months of crizotinib therapy for those with EML4-ALK fusion. The patients were randomised to either local consolidative therapy (LCT; surgery ± radiation to primary and metastases followed by standard maintenance or surveillance according to the physician’s choice) or no LCT (standard maintenance or surveillance according to the physician’s choice). PFS was defined as the primary outcome. Twenty-four patients were evaluable in each group.
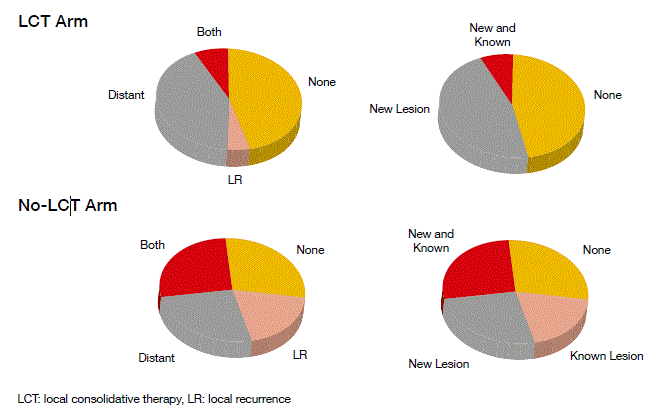
Patients treated with LCT fared significantly better than the no-LCT group. Median PFS was 11.9 vs. 3.9 months, respectively (p = 0.005). At the same time, toxicity did not differ substantially. There were differences in patterns of failure that trended towards significance (p = 0.09) (Figure). Patients in the no-LCT arm experienced a comparatively higher proportion of locoregional-only and known (vs. new-site) failures, whereas those in the LCT arm showed comparatively higher percentages of metastatic-only and new failures. Both locoregional and metastatic failures were more common in the no-LCT Arm (29 % vs. 8 %). The time to new-site failure significantly favoured LCT (11.9 vs. 5.7 months; p = 0.0497), which suggests reductions in the metastatic spread.
Figure: Differences in the patterns of failure by treatment arm (local consolidative therapy or no local consolidative therapy)
In the entire cohort, two other factors associated with PFS were identified: patients with two to three metastases after FLST had worse outcomes than those with only one lesion (p = 0.043), as did those without EGFR/ ALK alterations as compared to patients who were either EGFR-positive or ALK-positive (p = 0.035). Median OS was not reached in either arm. As the data are not yet mature, patients continue to be followed for this endpoint.
REFERENCES
- Gomez D et al., Local consolidative therapy (LCT) improves progression-free survival (PFS) in patients with oligometastatic non-small cell lung cancer (NSCLC) who do not progress after front line systemic therapy (FLST): results of a multiinstitutional phase II randomized study. J Clin Oncol 34, 2016 (suppl; abstr 9004)