ULTIMATE: chemotherapy plus bevacizumab beyond first line
As chemotherapy in the second-line or third-line settings of NSCLC shows limited efficacy, the phase III, randomised ULTIMATE trial tested the combination of chemotherapy and bevacizumab in patients with advanced NSCLC of non-squamous histology, who had progressed after one or two lines of treatment. Prior platinum-based and pemetrexed therapies were mandatory, and prior bevacizumab was allowed. While the control patients received docetaxel every three weeks (n = 55), those in the experimental arm were treated with paclitaxel weekly plus bevacizumab every four weeks (n = 109). Treatment continued until progression or toxicity.
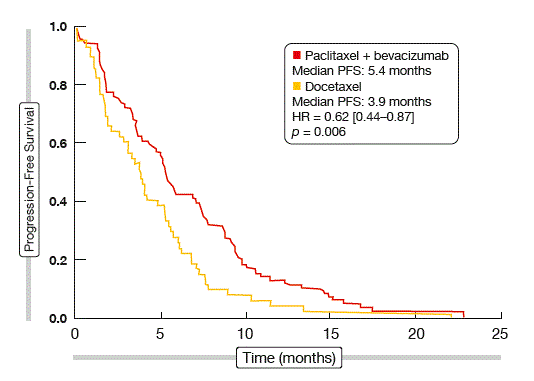
Weekly paclitaxel plus bevacizumab showed highly significant superiority over docetaxel monotherapy for both ORR at week 8 (22.5 % vs. 5.5 %; p = 0.006) and median PFS (5.4 vs. 3.9 months; p = 0.006). With the addition of bevacizumab, the risk of progression or death was reduced by 38 %. The PFS curves separated early on (Figure). According to the subgroup analysis, only patients with prior exposure to bevacizumab and those with performance status score of 2 did not benefit from the combined treatment. OS was similar across the two groups.
Figure: Progression-free survival with paclitaxel plus bevacizumab versus docetaxel
At the same time, the bevacizumab-based regimen showed significantly less haematological toxicity compared to docetaxel, and the patient quality of life was preserved. As the authors concluded, ULTIMATE introduces weekly paclitaxel and bevacizumab as a new second-line or third-line treatment option for NSCLC patients with non-squamous tumours.
REFERENCES
- Cortot AB et al., Weekly paclitaxel plus bevacizumab versus docetaxel as second or third-line treatment in advanced non-squamous non-small cell lung cancer (NSCLC): results from the phase III study IFCT-1103 ULTIMATE. J Clin Oncol 34, 2016 (suppl; abstr 9005)