ULTIMATE: chemotherapy plus bevacizumab beyond first line
As chemotherapy in the second-line or third-line settings of NSCLC shows limited efficacy, the phase III, randomised ULTIMATE trial tested the combination of chemotherapy and bevacizumab in patients with advanced NSCLC of non-squamous histology, who had progressed after one or two lines of treatment. Prior platinum-based and pemetrexed therapies were mandatory, and prior bevacizumab was allowed. While the control patients received docetaxel every three weeks (n = 55), those in the experimental arm were treated with paclitaxel weekly plus bevacizumab every four weeks (n = 109). Treatment continued until progression or toxicity.
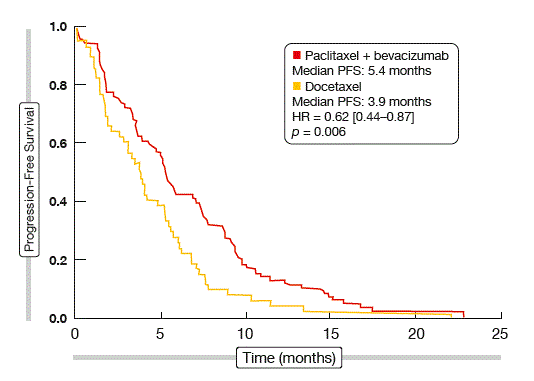
Weekly paclitaxel plus bevacizumab showed highly significant superiority over docetaxel monotherapy for both ORR at week 8 (22.5 % vs. 5.5 %; p = 0.006) and median PFS (5.4 vs. 3.9 months; p = 0.006). With the addition of bevacizumab, the risk of progression or death was reduced by 38 %. The PFS curves separated early on (Figure). According to the subgroup analysis, only patients with prior exposure to bevacizumab and those with performance status score of 2 did not benefit from the combined treatment. OS was similar across the two groups.
Figure: Progression-free survival with paclitaxel plus bevacizumab versus docetaxel
At the same time, the bevacizumab-based regimen showed significantly less haematological toxicity compared to docetaxel, and the patient quality of life was preserved. As the authors concluded, ULTIMATE introduces weekly paclitaxel and bevacizumab as a new second-line or third-line treatment option for NSCLC patients with non-squamous tumours.
REFERENCES
- Cortot AB et al., Weekly paclitaxel plus bevacizumab versus docetaxel as second or third-line treatment in advanced non-squamous non-small cell lung cancer (NSCLC): results from the phase III study IFCT-1103 ULTIMATE. J Clin Oncol 34, 2016 (suppl; abstr 9005)
More posts
Exploring established and novel EGFR-directed agents
The phase IIb LUX-Lung 7 trial was a head-to-head comparison of the second- generation ErbB family blocker afatinib and the first-generation reversible EGFR TKI gefitinib in patients with treatment-naïve, EGFR-mutation-positive, advanced (stage IIIB/IV) adenocarcinoma of the lung.According to the primary analysis, patients treated with afatinib derived significant PFS, ORR and time-to-treatment-failure benefits compared to those who received gefitinib.
Lung cancer care in Latin America: evolution of modern therapies and challenges to overcome the existing gaps
It is important to understand that Latin America is a large continent with approximately 600 million inhabitants. The four most-populated cities are Mexico City, Rio de Janeiro, São Paulo, and Buenos Aires, where the number of people living in just these four cities is equal to the total number of inhabitants of France.
Expanding treatment options in NSCLC patients with rare mutations: ALK, ROS1, MET, BRAF
ALK gene rearrangements occur in approximately 4 %to 5 % of all Caucasian and Asian patients with advanced NSCLC. Crizotinib was the first approved ALK inhibitor and is the current front-line standard treatment for ALK-positive NSCLC. However, despite initial responses to TKI treatment, all of these patients relapse in the long run.
Immunotherapy: updates on clinical trials and other insights
Besides targeted drugs for driver mutations, immunotherapies represent one of the two recent major advancements of the past decade for the treatment of metastatic non–small-cell lung cancer (NSCLC). Nivolumab and ipilimumab enhance T-cell antitumour activity through distinct and complementary mechanisms.
Preface – ASCO 2016
Lung cancer mortality rates for both men and women have been declining in recent years. Early detection, refined understanding of tumour biology, and a variety of novel treatment options have made these advances possible. Nevertheless, lung cancer is still the leading cause of cancer death in the United States and worldwide, prompting the scientific community to persevere in their research efforts and to extend them to areas that have traditionally been marked by little progress, such as small-cell lung cancer (SCLC).