EGFR TKI therapy in specific populations and settings
The first-generation EGFR TKIs erlotinib and gefitinib as well as the second-generation EGFR TKI afatinib have become the standard first-line treatment options for advanced EGFR-mutation–positive NSCLC. All three drugs improved PFS and objective response rate (ORR) compared to chemotherapy in phase III studies [1-4]. Afatinib induced prolongation of OS versus chemotherapy in patients with deletion 19 in the LUX-Lung 3 and 6 phase III studies [5]. In the phase II LUX-Lung 7 trial, afatinib, compared to gefitinib, gave rise to improvements in PFS, ORR, and time to treatment failure. [6]
Post-hoc analyses of the LUX-Lung trials
According to an analysis of the LUX-Lung 3 and 6 trials, tolerability-guided dose adjustment of afatinib is an effective measure to reduce treatment-related AEs without affecting therapeutic efficacy [7]. It diminished the interpatient variability of afatinib exposure and decreased the incidence and severity of AEs, while efficacy outcomes were similar across patients with and without dose reductions. Efficacious plasma levels were maintained, and patient-reported outcomes (PROs) did not change to a clinically meaningful extent.
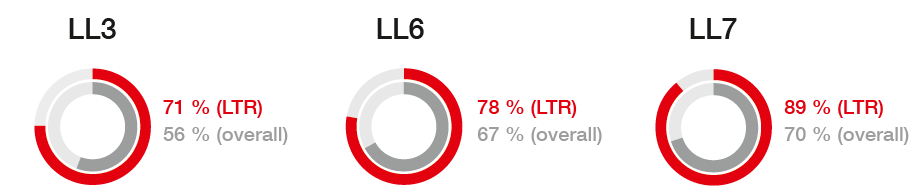
Schuler et al. performed a post-hoc analysis of afatinib long-term responders (LTRs) in the LUX-Lung 3, 6 and 7 studies [8]. In these three trials, 10 %, 10 % and 12 % of afatinib-treated patients, respectively, were LTRs. This equalled a total population of 66 individuals. Median treatment duration was 50, 56 and 42 months, respectively. Baseline patient characteristics were generally consistent with the overall study populations, with the exception of greater proportions of women and patients with deletion 19 among LTRs.
Median OS could not be estimated due to few deaths. Ranging from 71 % to 89 %, ORRs were higher in LTRs than in the overall LUX-Lung 3, 6 and 7 populations (Figure 1). Five patients (8 %) experienced CR. PR occurred in 47 patients (71 %) and SD in nine patients (14 %). LTRs tolerated afatinib treatment well. Long-term treatment was independent of tolerability-guided dose adjustment or baseline brain metastases. Also, it had no detrimental impact on subsequent therapies, which resembled those in the overall study populations. Likewise, PROs appeared stable between weeks 24 and 160; they even improved slightly after approximately 3 years of afatinib treatment compared to the start of therapy.
Figure 1: Response rates in the afatinib-treated overall populations of the LUX-Lung 3, 6 and 7 trials, and in long-term responders (LTRs) in these studies
Real-world data of afatinib in a large-scale Asian population
A large phase IIIb, open-label study is currently evaluating afatinib treatment in a broad Asian population of EGFR-TKI–naïve patients with locally advanced or metastatic EGFR-mutated NSCLC. An interim analysis of the data of 479 patients enrolled in five Asian countries was presented at the WCLC [9]. Two thirds of the population were chemotherapy-naïve. Thirty percent had received one prior line of chemotherapy, and 10 % had received ≥ 2 prior lines. Common mutations (deletion 19 and L858R mutation) were found in 86.0% of patients. Almost 20 % had asymptomatic brain metastases.
Median time to symptomatic progression (TTSP) and PFS in the overall cohort were 15.3 and 12.1 months, respectively. The fact that TTSP was 3 months longer than PFS suggests that afatinib therapy can be continued beyond progression, reflecting real-world clinical practice and treatment guidelines. TTSP and PFS were encouraging in patients with both common and uncommon EGFR mutations and in those with and without prior chemotherapy.
The safety data were consistent with those from the LUX-Lung 3, 6 and 7 studies. However, dose reductions occurred less often, confirming that in real-world practice, most afatinib-related AEs are manageable and result in few treatment discontinuations.
Efficacy in brain metastases and uncommon mutations
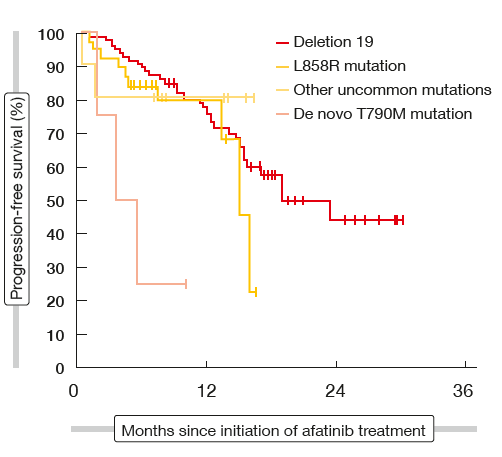
Likewise, in a retrospective Korean real-world analysis of 165 patients, first-line afatinib showed similar or even better PFS and OS outcomes compared with the clinical trials [10]. Median PFS was 19.1 months, and median OS had not been reached. At 12 and 24 months, 91.0 % and 70.7 % of patients were alive, respectively. The data also demonstrated the efficacy of afatinib in patients who had brain metastases before initiation of treatment. This cohort constituted almost half of the population (43.0 %). In those without any CNS irradiation, PFS was 15.7 months, which resembled PFS in patients who underwent Gamma Knife surgery (15.6 months). Those with whole brain radiotherapy experienced a median PFS of 11.5 months. Overall, CNS response rate was 75.9 %. In addition, the data showed that tumours harbouring uncommon EGFR mutations other than T790M also responded to afatinib treatment (Figure 2). In these patients, median PFS had not been reached at the time of the analysis. Compared to the clinical trials, more patients required dose reductions due to AEs, but this did not affect efficacy outcomes.
A case report underscores the activity of afatinib in uncommon mutations [11]. Lorandi et al. described the case of a 39-year-old female patient with adenocarcinoma of the lung that had already spread extensively to the bone and lymph nodes. Testing revealed an insertion of exon 20. While patients with deletion 19 and L858R point mutation generally benefit from TKI therapy, those with other mutations such as exon 20 insertions do not. However, the patient was offered afatinib treatment after she had received platinum-based chemotherapy and requested an alternative treatment, as she was not inclined to accept chemotherapy maintenance. After the initiation of afatinib treatment, the patient achieved a long-lasting partial response.
Figure 2: Activity of afatinib in patients with common and uncommon EGFR mutations
Potential combinations
Based on preclinical data suggesting a synergistic effect of afatinib and the anti-VEGF antibody bevacizumab [12], Kuyama et al. conducted a phase I trial evaluating afatinib plus bevacizumab as first-line treatment in 19 chemotherapy-naïve patients with advanced EGFR-mutant NSCLC [13]. Afatinib was tested at two dose levels (40 mg/day or 30 mg/day).
The analysis identified afatinib 30 mg/day and bevacizumab 15 mg/kg as the recommended regimen. This combination therapy was well tolerated and showed evidence of clinical activity. The ORR was 81.3 % for 16 evaluable patients, and all of them achieved disease control.
Another phase I trial assessed the combination of afatinib with carboplatin and pemetrexed in patients with EGFR-mutant metastatic NSCLC who had developed progression after first-line EGFR TKI treatment with gefitinib or erlotinib [14]. The combined administration of afatinib 20 mg/d (days 8 to 18) and pemetrexed 500 mg/m2 plus carboplatin AUC 5 (on day 1 every 21 days) demonstrated clinical activity. Median PFS was 16.2 months, and and disease control rate (DCR) was 100 %.
Dacomitinib: activity by EGFR mutation subtype
The randomised, open-label, phase III ARCHER 1050 trial tested the investigational second-generation EGFR TKI dacomitinib in the first-line setting. Patients with advanced EGFR-mutated NSCLC received either dacomitinib or gefitinib. Brain metastases were not allowed in this trial. Compared to gefitinib, dacomitinib showed significantly improved PFS (14.7 vs. 9.2 months; p < 0.0001) [15].
A prospective subgroup analysis of the ARCHER 1050 study assessing the activity of treatment by EGFR mutation subtype showed that dacomitinib was effective in patients with both exon 19 deletions and L858R mutations [16]. Compared to gefitinib, PFS was prolonged in both patient populations (exon 19 deletion, 16.5 vs. 9.2 months; HR, 055; p < 0.0001; L858R mutation, 12.3 vs. 9.8 months; HR, 0.63; p = 0.0034). While ORRs were comparable across EGFR TKI treatment (i.e., approximately 70 % in all cohorts), patients receiving dacomitinib achieved significantly longer duration of response in both genetic subgroups (exon 19 deletion, 15.6 vs. 8.3 months; HR, 0.454; p < 0.0001; L858R mutation, 13.7 vs. 7.5 months; HR, 0.403; p < 0.0001).
REFERENCES
- Rosell R et al., Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239-246
- Mok TS et al., Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947-957
- Wu YL et al., Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet On-col 2014; 15: 213-222
- Sequist LV et al., Phase III study of afatinib or cisplatin plus pemetrexed in patients with meta-static lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327-3334
- Yang JC-H et al., Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141-151
- Park K et al., Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17: 577-589
- Hirsh V et al., Afatinib dose adjustment: effect on safety, efficacy and patient-reported outcomes in the LUX-Lung 3/6 trials in EGFRm+ NSCLC. WCLC 2017 P3.01-075
- Schuler M et al., Analysis of long-term response to first-line afatinib in the LUX-Lung 3, 6 and 7 trials in advanced EGFRm+ NSCLC. WCLC 2017, P3.01-026
- Wu Y-L et al., A phase IIIb open-label, single-arm study of afatinib in EGFR-TKI-naïve patients with EGFRm+ NSCLC: An interim analysis. WCLC 2017, P3.01-036
- Kim Y et al., First-line afatinib for non-small cell lung cancer in real-world practice. WCLC 2017, P3.01-023
- Lorandi V et al., A case of a patient harboring an EGFR insertion of exon 20 and long lasting clinical response to afatinib. WCLC 2017, P2.03-029
- Ninomiya T et al., Afatinib prolongs survival compared with gefitinib in an epidermal growth factor receptor-driven lung cancer model. Mol Cancer Ther 2013; 12(5): 589-597
- Kuyama S et al., A phase I trial of afatinib and bevacizumab in untreated patients with advanced NSCLC harboring EGFR mutations: OL-CSG1404. WCLC 2017, P1.03-038
- Yamaguchi Ou et al., A phase I study evaluating the combination of afatinib, carboplatin and pemetrexed after failure of 1st generation EGFR TKIs. WCLC 2017, P2.03-021
- Mok TS et al., Dacomitinib versus gefitinib for the first-line treatment of advanced EGFR mutation positive non-small cell lung cancer
(ARCHER 1050): a randomized, open-label phase III trial. J Clin Oncol 35, 2017 (suppl; abstr LBA9007) - Wu YL et al., First-line dacomitinib versus gefitinib in advanced non-small cell lung cancer with EGFR mutation subgroups. WCLC 2017, OA 05.01
More posts
Chemotherapy: new approaches, new settings
Current guidelines recommend postoperative platinum-based chemotherapy in completely resected NSCLC with nodal involvement (stage II-IIIA). However, survival outcomes remain limited, and compliance is lower than for adjuvant therapy in other neoplasms. There are no direct comparisons between different chemotherapy regimens.
Malignant mesothelioma: recent data on nintedanib and checkpoint inhibitors
Malignant pleural mesothelioma (MPM) is an aggressive tumour that, if left untreated, shows a median survival of 7–9 months. The front-line standard treatment for patients with unresectable MPM consists of combination doublet therapy with cisplatin and pemetrexed, which yields a median OS of approximately 1 year.
Approaching squamous-cell carcinoma in a targeted manner
The EGFR mutation status is not routinely examined in NSCLC patients with squamous cell cancer (SCC) histology due to the low incidence of EGFR mutations in these tumours and poor clinical response to first-generation EGFR TKI treatment. Taniguchi et al. retrospectively reviewed 441 consecutive patients in 23 of whom the EGFR mutation status was assessed, in order to explore the clinical features of SCC with sensitive EGFR mutation, and to select the optimal indications for afatinib treatment.
Immunotherapy: novel biomarkers on the horizon & news from pivotal trials
Only limited treatment options are available for patients with recurrent small-cell lung cancer (SCLC). The CheckMate 032 trial evaluated the anti-PD-1 antibody nivolumab with or without the anti-CTLA-4 antibody ipilimumab in a PD-L1–unselected cohort of SCLC patients who had received at least one prior platinum-based chemotherapy regimen.
Taking anti-EGFR drug treatment further: later lines
Acquired resistance usually follows first-line EGFR TKI therapy, with the gatekeeper T790M mutation being the most common mechanism. The third-generation irreversible EGFR TKI osimertinib has been licensed for the treatment of patients whose tumours have been shown to carry this mutation. Retrospective data presented by Tan et al. demonstrated the activity of later-line osimertinib in 52 patients who participated in an early access program in Singapore.
“We are making steady progress toward better lung cancer control”
In a way, synergy is another expression for the multidisciplinary team approach, but the term ,multidisciplinary’ is not necessarily restricted to medical doctors. It also includes nursing staff and others such as the supportive care team, including the rehabilitation team and patient advocates. At the same time, the bottom line of that concept is having the patient at the centre of the overall care plan.