Taking anti-EGFR drug treatment further: later lines
Osimertinib after prior EGFR TKI therapy
Acquired resistance usually follows first-line EGFR TKI therapy, with the gatekeeper T790M mutation being the most common mechanism. The third-generation irreversible EGFR TKI osimertinib has been licensed for the treatment of patients whose tumours have been shown to carry this mutation. Retrospective data presented by Tan et al. demonstrated the activity of later-line osimertinib in 52 patients who participated in an early access program in Singapore [1]. Osimertinib was administered after progression on prior EGFR TKI therapy, from the second through the ninth treatment line (median, third line). Fiftythree percent of patients had brain metastases at initiation of treatment.
The independently assessed ORR was 46 %, with a median duration of response of 8.7 months. Complete responses (CRs) and partial responses (PRs) were achieved in 7.7 % and 38.5 %, respectively. Stable disease occurred in 40.4 %. Median PFS was 10.3 months; OS data were not mature at the time of the analysis. Osimertinib showed efficacy beyond the second line of therapy as well as regardless of the presence of CNS metastases.
Data on CNS control
Osimertinib is known to be CNS-active, which was confirmed by analyses presented at the WCLC. Zhu et al. assessed the efficacy of osimertinib 80 mg after first-generation TKI therapy in 10 patients with symptomatic brain lesions [2]. Two patients achieved PR in the CNS, and seven obtained stable disease (SD). Similarly, second-line osimertinib therapy exerted significant CNS control in Korean patients with measurable baseline brain metastases who participated in the open-label, multinational, real-world ASTRIS treatment study [3]. In the group of 16 patients evaluable for response, intracranial ORR was 81.3 %, with all of the patients achieving PR. The median duration of intracranial response had not been reached yet. Osimertinib showed clinical CNS efficacy irrespective of radiation history.
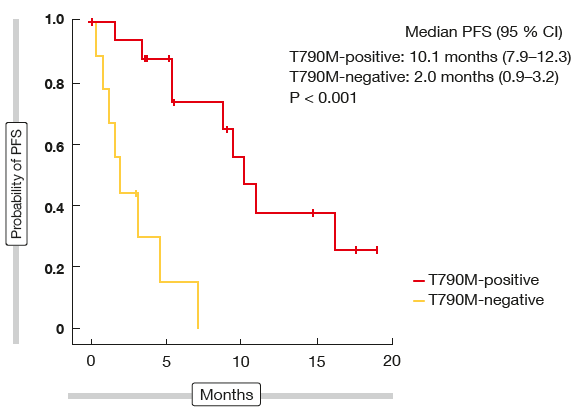
The single-arm, phase II TREM trial assessed the activity of osimertinib in T790M-positive and T790M-negative patients who had progressed after at least one EGFR TKI [4]. Thirty-four patients with brain metastases were included. The results indicated that osimertinib has similar efficacy in patients with CNS disease as in those without, whereas the benefit in T790M-negative patients appeared to be substantially lower. Overall, 75 % of patients experienced disease control, but this percentage was considerably higher in the T790M-positive cohort than in the T790M-negative group (88 % vs. 38 %). PFS was 10.1 vs. 2.0 months in these two cohorts (p < 0,001; Figure 1), while there was no significant PFS difference between the patients with brain metastases and those without (7.2 vs. 9.7 months; p = 0.300).
Figure 1: Osimertinib in patients with brain metastases: progression-free survival according to T790M mutation status
Prevalence of T790M mutation after afatinib
In patients who acquired resistance to first-line treatment with erlotinib and gefitinib, the T790M mutation showed prevalence rates of 49 % to 69 % [5-7]. However, data on resistance mechanisms to afatinib are lacking, particularly in Caucasian patients. Available evidence suggests that the development of the T790M mutation is also the predominant mechanism of afatinib resistance, with rates of 48 % to 68 % [7, 8].
In their single-centre, retrospective analysis, Hochmair et al. assessed the prevalence of the EGFR T790M mutation in patients who had progressed on afatinib treatment, as well as the response to osimertinib in this group [9]. Osimertinib has shown favourable results as second-line treatment after failure of first-generation or second-generation EGFR TKI therapy in the AURA3 study, but only 7 % of patients included in this trial had received first-line afatinib [10]. At the same time, emerging data suggest favourable clinical outcomes in patients who are prescribed the sequence of afatinib followed by osimertinib. According to a retrospective analysis of the LUX-Lung 3, 6 and 7 trials, median duration of osimertinib treatment after failure of afatinib was 20.2 months, and median OS had not yet been reached [11].
Consistent mutation rate & excellent response
Forty-eight patients who had progressed after initially achieving ≥ 3 months’ disease control with afatinib were included in this analysis. In 75 %, afatinib had been used in the first-line setting, whereas 19 % and 6 % of patients, respectively, had received the TKI as a second-line or third-line agent. Testing showed that 56 % (n = 27) had developed the EGFR T790M mutation, which is consistent with the available prevalence rates from previous analyses [7, 8] and the T790M mutation rates in patients who progressed on erlotinib or gefitinib treatment [5–7].
Additional tissue rebiopsy was performed in 34 patients to confirm liquid biopsy findings, giving a concordance rate of 91 % between the two tests. Emergence of the T790M mutation did not appear to correlate with baseline characteristics or other parameters such as the duration of response to afatinib. For patients receiving afatinib in the second or third line, it is not known when the T790M mutation emerged, as testing took place only after failure of afatinib therapy.
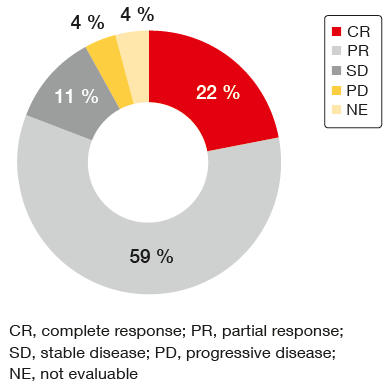
In the 27 patients who had developed T790M mutation, treatment with osimertinib elicited a high ORR of 81 %, with 22 % of patients achieving CR (Figure 2). Data on the duration of response to osimertinib were immature at the time of the analysis. Osimertinib treatment was ongoing in 11 (41 %) of patients. Median time on sequential treatment with afatinib and osimertinib was 25.0 months.
Figure 2: Response to osimertinib in patients who developed T790M mutation after initially achieving ≥ 3 months’ disease control with afatinib
Mechanisms of resistance to osimertinib
Based on the phase III FLAURA trial, osimertinib is an emerging standard of care for the first-line treatment of metastatic EGFR-mutation–positive NSCLC [12]. However, acquired resistance to osimertinib represents a challenge, even more so as it has not been systematically characterised to date. Understanding the mechanisms of resistance to third-generation EGFR TKIs is pivotal for the future development of next-generation EGFR TKIs and drug combinations.
Therefore, Puri et al. retrospectively reviewed the genomic profiles of 51 patients with metastatic NSCLC and T790M mutation to identify the potential mechanisms of resistance to osimertinib [13]. Among the 51 patients, 35 had been treated with osimertinib; as expected, they showed significantly longer OS than the group of 16 patients who had not received osimertinib (25.8 vs. 4.34 months; p = 0.019). According to the genomic profiling of 10 patients who developed progressive disease on osimertinib, EGFR-dependent mechanisms, such as C797S or C797G mutation, loss of EGFR T790M and EGFR amplification, were most common (80 %). In addition, EGFR-independent mechanisms occurred in 60 %. These included HER2 and MET amplification, activation of accessory pathways (e.g., MAPK/ERK pathway), and others (e.g., RET NCOA4 fusion, MYC amplification). Each patient showed multiple mechanisms of resistance at the time of genomic testing.
Loss of T790M does not indicate resensitisation
Oxnard et al. also focussed on describing mechanisms of resistance to osimertinib [14]. The scientists performed tumour and plasma genotyping from patients who received single-agent osimertinib for T790M-positive NSCLC after acquired resistance to prior EGFR TKI treatment, using plasma from the AURA trial for purposes of validation. Among 33 patients who progressed on osimertinib treatment, 11 maintained T790M, and 22 lost it. The EGFR C797S mutation, which is deemed characteristic of osimertinib-resistant tumours, was detected only in patients who maintained T790M mutation. In those who lost it, competing resistance mechanisms occurred, including histologic transformation to SCLC, MET amplification or PIK3CA mutation. Patients with loss of T790M showed early resistance to osimertinib; here, median time to treatment failure was 6.9 months. In contrast, resistance due to the C797S mutation is frequently observed later on in the treatment course. At the same time, loss of T790M mutation is difficult to predict from baseline plasma genotyping. The relative T790M allelic fraction was only slightly lower for patients with loss of T790M than for those with maintained T790M.
The authors concluded that loss of T790M does not indicate resensitisation to first-generation EGFR TKI treatment, but often indicates overgrowth of a competing resistance mutation. The range of rare genetic resistance mechanisms to look out for includes KRAS mutations, RET fusions, and EGFR fusions. Retesting for T790M at progression might help to elucidate the biology of resistance. The authors suggested considering a trial of osimertinib combined with alternate pathway inhibitors (e.g., a MET inhibitor) in case of early resistance; for patients with late resistance, a study of osimertinib plus an additional EGFR inhibitor might be appropriate, as resistance with maintained EGFR addiction can be suspected.
REFERENCES
- Tan WL et al., Clinical outcomes of patients with EGFR T790M+ NSCLC on osimertinib. WCLC 2017, P3.01-017
- Zhu L et al., The feasibility of osimertinib treatment on brain metastases in NSCLC patients after 1st generation EGFR-TKI resistance: a preliminary study. WCLC 2017, P1.01-046
- Kim JH et al., Efficacy of osimertinib for CNS metastases in advanced NSCLC: data from a Korea single center in ASTRIS, a real world treatment study. WCLC 2017, P3.01-028
- Zwicky Eide IJ et al., Osimertinib in relapsed EGFR-mutated non-small cell lung cancer patients with brain metastases: results from the TREM-Study. WCLC 2017, P2.03-035
- Yu HA et al., Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI ther-apy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19: 2240-2247
- Sequist LV et al., Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3: 75ra26
- Yang JC-H et al., Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 2017; 35: 1288-1296
- Wu SG et al., The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget 2016; 7: 12404-12413
- Hochmair MJ et al., Prevalence of EGFR T790M mutation in NSCLC patients after afatinib failure, and subsequent response to osimertinib. WCLC 2017, P2.03-025
- Mok TS et al., Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376: 629-640
- Sequist LV et al., Subsequent therapies post-afatinib among patients with EGFR mutation-positive NSCLC in LUX-Lung (LL) 3, 6 and 7. ESMO 2017 Congress, abstract 1349P
- Ramalingam SS et al., Osimertinib vs. standard-of-care EGFR-TKI as first-line treatment in patients with EGFRm advanced NSCLC: FLAURA. ESMO 2017 Congress, abstract LBA2_PR
- Puri S et al., Genomic profiling of EGFR T790M mutated non-small cell lung cancer to evaluate the mechanisms of resistance to osimertinib. WCLC 2017, MA 12.05
- Oxnard GR et al., Osimertinib resistance mediated by loss of EGFR T790M is associated with early resistance and competing resistance mechanisms. WCLC 2017, OA 09.02
More posts
Chemotherapy: new approaches, new settings
Current guidelines recommend postoperative platinum-based chemotherapy in completely resected NSCLC with nodal involvement (stage II-IIIA). However, survival outcomes remain limited, and compliance is lower than for adjuvant therapy in other neoplasms. There are no direct comparisons between different chemotherapy regimens.
Malignant mesothelioma: recent data on nintedanib and checkpoint inhibitors
Malignant pleural mesothelioma (MPM) is an aggressive tumour that, if left untreated, shows a median survival of 7–9 months. The front-line standard treatment for patients with unresectable MPM consists of combination doublet therapy with cisplatin and pemetrexed, which yields a median OS of approximately 1 year.
Approaching squamous-cell carcinoma in a targeted manner
The EGFR mutation status is not routinely examined in NSCLC patients with squamous cell cancer (SCC) histology due to the low incidence of EGFR mutations in these tumours and poor clinical response to first-generation EGFR TKI treatment. Taniguchi et al. retrospectively reviewed 441 consecutive patients in 23 of whom the EGFR mutation status was assessed, in order to explore the clinical features of SCC with sensitive EGFR mutation, and to select the optimal indications for afatinib treatment.
Immunotherapy: novel biomarkers on the horizon & news from pivotal trials
Only limited treatment options are available for patients with recurrent small-cell lung cancer (SCLC). The CheckMate 032 trial evaluated the anti-PD-1 antibody nivolumab with or without the anti-CTLA-4 antibody ipilimumab in a PD-L1–unselected cohort of SCLC patients who had received at least one prior platinum-based chemotherapy regimen.
Taking anti-EGFR drug treatment further: later lines
Acquired resistance usually follows first-line EGFR TKI therapy, with the gatekeeper T790M mutation being the most common mechanism. The third-generation irreversible EGFR TKI osimertinib has been licensed for the treatment of patients whose tumours have been shown to carry this mutation. Retrospective data presented by Tan et al. demonstrated the activity of later-line osimertinib in 52 patients who participated in an early access program in Singapore.
“We are making steady progress toward better lung cancer control”
In a way, synergy is another expression for the multidisciplinary team approach, but the term ,multidisciplinary’ is not necessarily restricted to medical doctors. It also includes nursing staff and others such as the supportive care team, including the rehabilitation team and patient advocates. At the same time, the bottom line of that concept is having the patient at the centre of the overall care plan.