Anti-angiogenesis with nintedanib: activity in mesothelioma, and potential biomarkers
LUME-Meso
Malignant pleural mesothelioma generally has poor patient prognosis, as it is often diagnosed at an advanced stage. The only approved regimen consists of the combination of pemetrexed and cisplatin, which gives rise to a median OS of approximately 1 year [1]. The randomised, double-blind, placebo-controlled, phase II LUME-Meso trial tested the oral multikinase inhibitor nintedanib for treatment of mesothelioma. Nintedanib targets pro-angiogenic pathways mediated by VEGF1-3, FGFR1-3 and PDGFRα/β, as well as the kinases Src and Abl; all of these are involved in the pathogenesis of mesothelioma [2, 3]. In contrast to other drugs of the same class, nintedanib can be safely combined with commonly used chemotherapy. Nintedanib has demonstrated efficacy in in-vitro and in-vivo models of mesothelioma [4].
The LUME-Meso trial evaluated the addition of nintedanib 200 mg BID to the standard chemotherapy of pemetrexed/ cisplatin (n = 44) compared to placebo plus pemetrexed/ cisplatin alone (n = 43), in patients with unresectable malignant pleural mesothelioma who had not received prior chemotherapy. Patients who did not develop disease progression were put on nintedanib maintenance (experimental arm) or placebo maintenance (control arm) until progression. PFS was defined as the primary endpoint. This was an exploratory study, with all of the statistics intended to be descriptive.
Specific advantage in the epitheloid subtype
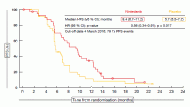
The addition of nintedanib to chemotherapy led to clinically meaningful PFS improvement of 3.7 months (9.4 vs. 5.7 months; HR, 0.56; p = 0.017; Figure) [5]. Almost all of the subgroups derived greater benefit from the combination. Nintedanib-treated patients also demonstrated improved response rate (59 %vs. 44 %) and a trend for extended OS (18.3 vs. 14.5 months; HR, 0.78), although the results are still immature here. Patients with epitheloid histology made up almost 90 % of the study population. In this group, PFS results were comparable to those in the total population, while OS showed further improvement (18.3 vs. 15.2 months; HR, 0.68), even though statistical significance was not reached.
The safety profile was consistent with that observed in previous combination studies, and predominantly featured diarrhoea and cytopenias. Grade ≥ 3 AEs occurred only infrequently. AEs commonly reported with VEGF/ VEGFR inhibitors, such as hypertension, bleeding or thromboembolism, were rare and well balanced between the two arms. The addition of nintedanib did not decrease the number of completed chemotherapy cycles or the dose intensity of the chemotherapy. Based on these data, the phase III part of the LUME-Meso confirmatory trial is currently recruiting patients. The study design is identical with the phase II part, but only patients with epitheloid histology are being enrolled.
Figure: LUME-Meso: PFS with nintedanib or placebo in addition to standard chemotherapy in patients with malignant pleural mesothelioma
Angiogenic factors and radiotracer imaging
Nintedanib plus docetaxel has been approved for the treatment of adenocarcinoma of the lung after failure of chemotherapy, although not all such patients benefit from this anti-angiogenic approach. Identification of predictive markers for response is therefore vital. A phase II trial assessed the correlation between plasma levels of VEGF, FGF and PDGF and clinical endpoints of DCR, PFS and OS in patients with NSCLC treated with nintedanib plus docetaxel [6]. Thirty-eight patients diagnosed with stage IIIB/IV adenocarcinoma of the lung who had developed progression after platinum-based first-line chemotherapy were included. This is the first trial to use angiogenic factors as biomarkers for response to nintedanib therapy in patients with NSCLC. The analysis yielded promising results, as levels of angiogenic factors, particularly FGF, correlated with longer OS. Also, the development of grade 1 hypertension was associated with PFS improvement.
In the same patient population, Arrieta et al. evaluated the use of PET/ computed tomography (CT) with the peptide radiotracer [68Ga]-DOTA-E-[c(RGDfK)]2 to measure the expression of αvβ3 integrin during angiogenesis in tumour tissue [7]. αvβ3 integrin is a molecular target for non-invasive monitoring of fast-growing malignant cells, as well as for assessment of treatment response. The results showed that larger baseline tumour volume was associated with longer PFS. A reduction in the percentage change (> 11.8 %) of the lung/ spleen maximum standardised uptake value index was associated with improved OS. According to the investigators, [68Ga]-DOTA-E-[c(RGDfK)]2 PET/ CT appears to be a more useful tool than [18F]-FDG PET/ CT for the assessment of response in NSCLC patients receiving treatment with nintedanib.
REFERENCES
- Vogelzang NJ et al., Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21(14): 2636- 2644
- Robinson BW & Lake RA, Advances in malignant mesothelioma. New Engl J Med 2005; 353(15): 1591-1603
- Khusial PR et al., Src activates Abl to augment Robo1 expression in order to promote tumor cell migration. Oncotarget 2010; 1(3): 198- 209
- Laszlo V et al., Preclinical investigation of the therapeutic potential of nintedanib in malignant pleural mesothelioma. WCLC 2015, ORAL14.07
- Grosso F et al., Nintedanib plus pemetrexed/ cisplatin in patients with MPM: phase II findings from the placebo-controlled LUME-Meso trial, WCLC 2016, OA22.02
- Lee-Cervantes D et al., Soluble angiogenic factors as predictive biomarkers of response to docetaxel plus nintedanib as second-line therapy in NSCLC. WCLC 2016, P2.03b-083
- Arrieta O et al., PET-CT with 68Ga-RGD as biomarker of response to nintedanib plus docetaxel as second-line therapy in NSCLC. WCLC 2016, P2.03b-088
More posts
Practice-changing refinements of lung cancer staging
The 8th edition of the TNM classification has recently come into effect. Compared to the 7th edition published in 2009, several important adjustments have been made to lung cancer staging with the aim of improving prognostication and research. “Research is of particular significance in the context of smaller tumours that can be treated with a variety of therapeutic options,” said Ramón Rami-Porta, MD, PhD, Department of Thoracic Surgery, Hospital Universitari Mútua Terrassa, Terrassa, Barcelona, Spain.
Anti-angiogenesis with nintedanib: activity in mesothelioma, and potential biomarkers
Malignant pleural mesothelioma generally has poor patient prognosis, as it is often diagnosed at an advanced stage. The only approved regimen consists of the combination of pemetrexed and cisplatin, which gives rise to a median OS of approximately 1 year. The randomised, double-blind, placebo-controlled, phase II LUME-Meso trial tested the oral multikinase inhibitor nintedanib for treatment of mesothelioma.
Who is a candidate for immunotherapy?
When we look at immunotherapy for NSCLC, we should realize that approximately 20 % of treated patients show a response. To direct treatment to patients with a higher likelihood of response, biomarkers might be of value. One biomarker that is established in clinical practice is PD-L1 expression on the tumour, according to immunohistochemistry.
Immunotherapy: novel anti-PD-L1 antibodies & various combination regimens
As compared to anti-PD-1 antibodies, the advantage of antibodies directed against PD-L1 is that they can inhibit PD-1/ PD-L1 interactions while leaving the PD-1/ PD-L2 pathway intact, thus potentially preserving peripheral immune homoeostasis. OAK was the first randomised phase III trial to assess an anti-PD-L1 agent in advanced NSCLC.
Liquid biopsy in the context of EGFR and other mutations
Compared to tissue biopsy and re-biopsy, liquid biopsy offers several advantages, including minimal-invasiveness, the opportunity for serial measurements over time to monitor tumour response, and detection of resistance mutations in the plasma prior to radiographic detection. The issue of tumour heterogeneity, which is an important factor in therapeutic failure, is also considered.
Emerging treatments in ALK-positive NSCLC: new options, but also new challenges
Treatment with the ALK tyrosine kinase inhibitor (TKI) crizotinib has been established as a standard first-line option in patients with ALK-rearranged advanced NSCLC. Before the advent of crizotinib, a platinum–pemetrexed doublet followed by pemetrexed maintenance was standard of care in non-squamous NSCLC. However, after an initial response to crizotinib, acquired resistance invariably develops due to multiple mechanisms, which can include secondary mutations in the ALK tyrosine kinase domain.