Combination of targeted therapy with radiotherapy for treatment of brain metastasis
Lung adenocarcinomas often metastasize to the brain, and the prognosis of patients with brain metastases is poor. The EGFR gene is mutated in a considerable fraction of patients with primary lung adenocarcinomas and brain metastases, and especially in Asian patients. As reported at CSCO 2017, the prevalence of EGFR mutation among these patients with brain metastases is about 44 % in Taiwan (China) and 63 % in Japan, which is dramatically higher than in America or Europe (at 0 %–2 %) [1]. At CSCO 2017, the main progress on treatment of these brain metastases focused on the efficacy of driver-oncogene-positive targeted therapy with or without radiotherapy, and the optimal sequence of radiotherapy with EGFR/ALK tyrosine kinase inhibitors (TKIs) in patients with driver-oncogene-positive non–small-cell lung cancer (NSCLC).
Targeted therapy for brain metastases in driver-oncogene-positive NSCLC
The first generation EGFR/ALK TKIs showed improved progression-free survival (PFS) in driver-oncogene-positive NSCLC patients with brain metastases. Kim JE et al. [2] conducted a study that enrolled EGFR-mutant-positive NSCLC patients with asymptomatic brain metastases. The patients were treated with gefitinib (250 mg) or erlotinib (150 mg) once daily as first-line treatment. The results showed that out of 23 patients, 16 achieved partial response (PR), three stable disease (SD), and four progressive disease. The median PFS and overall survival (OS) were 7.1 and 18.8 months, respectively [2].
The PROFILE 1005 and 1007 studies retrospectively analyzed the efficacy of the ALK and ROS1 inhibitor crizotinib in advanced ALK-rearranged NSCLC patients with previously untreated asymptomatic brain metastases. The results showed that systemic disease control rate (DCR) at 12 weeks was 63 %, intracranial DCR was 56 %, and median intracranial time to progression (TTP) was 7 months. Importantly, 20 % of the patients with newly developed progressive disease after initiation of crizotinib treatment were diagnosed with brain metastases. Thus, the first generation TKIs (including EGFR TKIs and ALK TKIs) are efficacious for treatment of driver-oncogene-positive NSCLC patients with brain metastases. However, only relatively short extensions of PFS, OS, and TTP, and relatively low DCR, were reached [3]. Additionally, the high proportion of patients who still developed brain metastases after ALK TKI treatment was not encouraging.
Central nervous system response to osimertinib
The emergence of osimertinib and alectinib opened new options for treatment of driver-oncogene-positive NSCLC patients with brain metastases. The AURA3 trial was the first comparative evidence for the efficacy of osimertinib versus platinum–pemetrexed in patients with metastases in the central nervous system (CNS). Osimertinib improved the objective response rate compared to chemotherapy (70 % vs. 31 %), with significantly improved PFS in the osimertinib group (10.1 vs. 4.4 months; hazard ratio [HR] after adjustment for Asian or non-Asian race, 0.30; 95 % confidence interval [CI], 0.23 to 0.41; p < 0.001) The HR for PFS favored osimertinib across all predefined subgroups that were analyzed, including patients with CNS metastases (median PFS, 8.5 vs. 4.2 months; HR, 0.32; 95 % CI, 0.21 to 0.49) [4].
Compared to these data for the first-generation EGFR TKI, the FLAURA trial then showed that in first-line treatment of EGFR-mutant-positive NSCLC with brain metastases, median PFS was improved for osimertinib versus first generation EGFR TKI (15.2 vs. 9.6 months; HR 0.47; 95 % CI 0.30–0.74; p < 0.001) [5].
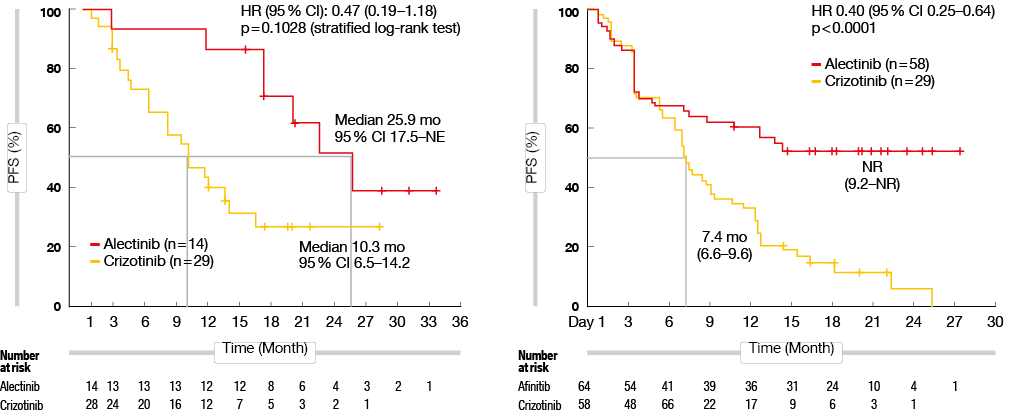
The recently published J-ALEX and ALEX studies compared the efficacy of alectinib versus crizotinib as first-line treatment for ALK-mutant-positive NSCLC patients. Alectinib was reported to show improved penetration of the blood brain barrier. Thus, alectinib treatment provided improved PFS for NSCLC patients with co-occurrence of CNS metastases, compared to crizotinib (J-ALEX: 25.9 months vs. 10.3 months, p = 0.1028; ALEX: not reached vs. 7.4 months, p < 0.0001) (Figure 1). Furthermore, the alectinib group showed lower 12-month cumulative CNS metastasis incidence rate, compared to the crizotinib group (16.0 % vs. 58.3 %) [6].
Figure 1: Progression-free survival for alectinib versus crizotinib for treatment of ALK-mutant-positive NSCLC patients with CNS metastasis at baseline in the J-ALEX (left) and ALEX (right) studies.
Strategies to fight brain metastasis – upfront radiosurgery versus radiation therapy versus tyrosine kinase inhibitors
Tyrosine kinase inhibitors have demonstrated efficacy against the incidence of brain metastases and have prolonged PFS in NSCLC patients with brain metastases. Additionally, whole brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS) have been used for treatment of NSCLC patients with brain metastases. WBRT is regarded as the standard treatment for tumors of large size, or for patients with more than three lesions. Common side effects include neurocognitive dysfunction, such as cognitive impairment and altered executive function. SRS is suitable for patients with better prognosis, and according to a report by Shultz DB et al. [7], multiple courses of SRS and deferring WBRT for distant brain metastases after initial SRS appears to be a safe and effective approach. To further prolong patient survival, combined SRS, WBRT and targeted therapy appears to be effective.
Magnuson WJ et al. [8] conducted a retrospective study that compared the efficacies of these treatments for EGFR-mutant NSCLC patients with intracranial progression: SRS followed by EGFR TKI; WBRT followed by EGFR TKI; and EGFR TKI followed by either SRS or WBRT. Their results showed that median OS for these SRS (n = 100), WBRT ( =120), and EGFR TKI (n = 131) cohorts was 46, 30, and 25 months, respectively (p < 0.001) [8]. The use of up-front SRS provided the best OS and showed lower potential neurocognitive sequelae for the WBRT. Finally, they also indicated that although upfront SRS appeared to be the best choice, in the era of targeted therapies there remain several uncertainties. Hence, a prospective phase III study is needed [1].
Optimal strategy to manage central nervous system metastases in driver-oncogene-positive NSCLC in China.
Yang JJ et al. [9] conducted a phase 3 study in China that compared the efficacies of the EGFR TKI icotinib and whole-brain irradiation (WBI) in patients with driver-oncogene-positive NSCLC and brain metastases. The results showed that median intracranial PFS was improved with icotinib compared to WBI (10 vs. 4.8 months; p = 0.014), and the rate of adverse events higher than grade 3 was lower in the icotinib group compared to the WBI treatment group (8 % vs. 38 %) [9]. Thus, they suggested that in China, EGFR TKIs should be the treatment of choice in EGFR-mutant-positive NSCLC patients with brain metastases.
REFERENCES
- Lu Y et al., Management of CNS Metastasis in Patients with Driver Oncogene Positive NSCLC. CSCO 2017
- Kim JE et al., Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors as a First-Line Therapy for Never-Smokers with Adenocarcinoma of the Lung Having Asymptomatic Synchronous Brain Metastasis. Lung Cancer. 2009 Sep;65(3):351-4.
- Costa DB et al., Clinical Experience With Crizotinib in Patients with Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol. 2015 Jun 10;33(17):1881-8.
- Mok TS et al., Osimertinib or Platinum–Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017 Feb 16;376(7):629-640.
- Soria JC et al., Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N Engl J Med. 2018 Jan 11;378(2):113-125. doi: 10.1056/NEJMoa1713137.
- Peters S et al., Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2017 Aug 31;377(9):829-838.
- Shultz DB et al., Repeat Courses of Stereotactic Radiosurgery (SRS), Deferring Whole-Brain Irradiation, for New Brain Metastases after Initial SRS. Int J Radiat Oncol Biol Phys. 2015 Aug 1;92(5):993-999
- Magnuson WJ et al., Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non–Small-Cell Lung Cancer: a Retrospective Multi-Institutional Analysis. J Clin Oncol. 2017 Apr 1;35(10):1070-1077.
- Yang JJ et al., Icotinib versus Whole-Brain Irradiation in Patients with EGFR-Mutant Non–Small-Cell Lung Cancer and Multiple Brain Metastases (BRAIN): a Multicentre, Phase 3, Open-Label, Parallel, Randomised Controlled Trial. Lancet Respir Med. 2017 Sep;5(9):707-716.