Who is a candidate for immunotherapy?
Johan Vansteenkiste, MD, PhD, Respiratory Oncology Unit/Pulmonology, University Hospital KU Leuven, Belgium
Which markers are available that define patients who are suitable for immunotherapy?
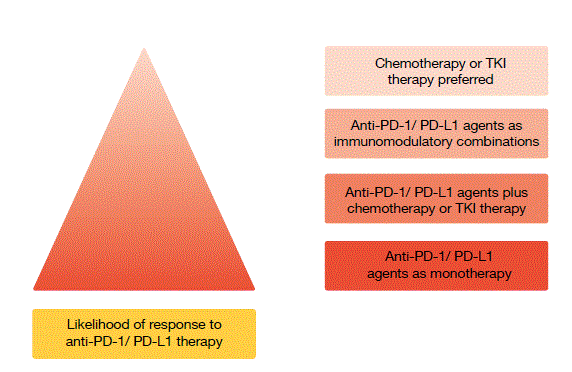
When we look at immunotherapy for NSCLC, we should realize that approximately 20 % of treated patients show a response. To direct treatment to patients with a higher likelihood of response, biomarkers might be of value. One biomarker that is established in clinical practice is PD-L1 expression on the tumour, according to immunohistochemistry. For the time being, there are different methodologies to assess PD-L1 expression, but they appear to coalesce slowly, so probably we will eventually have quite a reliable read-out based on immunohistochemistry. PD-L1 is not an absolute marker like the molecular aberrations that we are using for the prescription of targeted agents, but it is what is called an enrichment marker. This means that the higher the PD-L1 expression is, the higher the likelihood that the patient will respond to immunotherapy. Patients can be PD-L1–negative and still have a response, but these are only very few cases. Hence, PD-L1 expression helps to select patients for that type of expensive therapy, and it helps to select patients for immunotherapy when there are perhaps other treatment options available that might be preferable (Figure).
Figure: Likelihood of response to immunotherapy according to PD-L1 expression, and preferred therapeutic approaches
In which settings should immunotherapeutic approaches be avoided?
Immunotherapy certainly represents great progress in the treatment of NSCLC, but there are patients who are less suitable, or even who have contraindications to this type of therapy. General exclusion criteria include prior allogenic bone marrow transplantation or solid organ transplantation, as suppression of the immune system is of vital importance for those patients. Another contraindication is autoimmune disease or a history of autoimmune disease, because frequently these patients already receive immunosuppressive treatment. In these cases, stimulation of the immune system is of course not an option, and certainly not stimulation of the lymphocytes, which are often at the centre of the pathogenesis of autoimmune diseases. Furthermore, there are some other vulnerabilities that are less absolute, such as interstitial lung disease, active hepatitis, or conditions outside the autoimmune context that require systemic treatment with corticosteroids at daily doses of more than 10 mg prednisone equivalent. Cancer patients who require high doses of corticosteroids for the treatment of their brain metastases are a typical example.
How would you rate the global situation regarding practical restrictions, such as reimbursements and availability?
We have seen progress with immunotherapy in NSCLC, but most of these agents are expensive. Therefore, access to this therapy for patients is very variable across different regions of the world. In the more developed countries, we see that reimbursement for immunotherapy is gradually being implemented, but even developed nations can look very critically at the incremental value according to the incremental cost. For instance, in the United Kingdom, the national body, NICE, rejected immunotherapy for NSCLC because the cost is not in relation to the real extra value to the patient. Obviously, there is still a long way to go. We can only hope that once more agents are approved, there will be competition, which might decrease the cost of immunotherapy. This would create the possibility for access to this important therapy for an increasing number of patients and in an increasing number of countries.
Johan Vansteenkiste discusses the practical considerations for immunotherapy.
Johan Vansteenkiste at the WCLC congress
You are currently viewing a placeholder content from Default. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
Johan Vansteenkiste discusses the practical considerations for immunotherapy.
REFERENCES
- Pignon JP et al., Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008; 26(21): 3552-3559
- Chen L et al., Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014; 5: 5241
- Cascone T et al., Neoadjuvant nivolumab or nivolumab plus ipilimumab for resectable non-small cell lung cancer (NSCLC): clinical and correlative results from the NEOSTAR study. J Clin Oncol 37, 2019 (suppl; abstr 8504)
- Kwiatkowski DJ et al., Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 37, 2019 (suppl; abstr 8503)
- Provencio M et al., Neo-adjuvant chemo-immunotherapy for the treatment of stage IIIA resectable non-small-cell lung cancer (NSCLC): a phase II multicenter exploratory study. Final data of patients who underwent surgical assessment (NADIM). J Clin Oncol 37, 2019 (suppl; abstr 8509)
- Kenmotsu H et al., Randomized phase III study of pemetrexed/cisplatin versus vinorelbine/cisplatin for completely resected non-squamous non-small-cell lung cancer. The JIPANG study. J Clin Oncol 37, 2019 (suppl; abstr 8501)
- Tang W et al., EGFR inhibitors as adjuvant therapy for EGFR mutation positive non-small cell lung cancer. J Clin Oncol 37, 2019 (suppl; abstr 8508)
- Khalil M et al., The tumor microenvironment in EGFR-driven loco-regional lung adenocarcinoma can predict higher risk of recurrence. J Clin Oncol 37, 2019 (suppl; abstr 8521)
- Chaft JE et al., Randomized phase II study of adjuvant afatinib for 3 months versus 2 years in patients with resected stage I-III EGFR mutant NSCLC. J Clin Oncol 37, 2019 (suppl; abstr 8507)
- Moding EJ et al., ctDNA for personalization of consolidation immunotherapy in localized non-small cell lung cancer. J Clin Oncol 37, 2019 (suppl; abstr 2547)
© 2019 Springer-Verlag GmbH, Impressum
More posts
Practice-changing refinements of lung cancer staging
The 8th edition of the TNM classification has recently come into effect. Compared to the 7th edition published in 2009, several important adjustments have been made to lung cancer staging with the aim of improving prognostication and research. “Research is of particular significance in the context of smaller tumours that can be treated with a variety of therapeutic options,” said Ramón Rami-Porta, MD, PhD, Department of Thoracic Surgery, Hospital Universitari Mútua Terrassa, Terrassa, Barcelona, Spain.
Anti-angiogenesis with nintedanib: activity in mesothelioma, and potential biomarkers
Malignant pleural mesothelioma generally has poor patient prognosis, as it is often diagnosed at an advanced stage. The only approved regimen consists of the combination of pemetrexed and cisplatin, which gives rise to a median OS of approximately 1 year. The randomised, double-blind, placebo-controlled, phase II LUME-Meso trial tested the oral multikinase inhibitor nintedanib for treatment of mesothelioma.
Who is a candidate for immunotherapy?
When we look at immunotherapy for NSCLC, we should realize that approximately 20 % of treated patients show a response. To direct treatment to patients with a higher likelihood of response, biomarkers might be of value. One biomarker that is established in clinical practice is PD-L1 expression on the tumour, according to immunohistochemistry.
Immunotherapy: novel anti-PD-L1 antibodies & various combination regimens
As compared to anti-PD-1 antibodies, the advantage of antibodies directed against PD-L1 is that they can inhibit PD-1/ PD-L1 interactions while leaving the PD-1/ PD-L2 pathway intact, thus potentially preserving peripheral immune homoeostasis. OAK was the first randomised phase III trial to assess an anti-PD-L1 agent in advanced NSCLC.
Liquid biopsy in the context of EGFR and other mutations
Compared to tissue biopsy and re-biopsy, liquid biopsy offers several advantages, including minimal-invasiveness, the opportunity for serial measurements over time to monitor tumour response, and detection of resistance mutations in the plasma prior to radiographic detection. The issue of tumour heterogeneity, which is an important factor in therapeutic failure, is also considered.
Emerging treatments in ALK-positive NSCLC: new options, but also new challenges
Treatment with the ALK tyrosine kinase inhibitor (TKI) crizotinib has been established as a standard first-line option in patients with ALK-rearranged advanced NSCLC. Before the advent of crizotinib, a platinum–pemetrexed doublet followed by pemetrexed maintenance was standard of care in non-squamous NSCLC. However, after an initial response to crizotinib, acquired resistance invariably develops due to multiple mechanisms, which can include secondary mutations in the ALK tyrosine kinase domain.