SCLC: prognostic determinants and new treatment modalities
Long-term survival in CASPIAN
The global, randomized, open-label, phase III CASPIAN trial was initiated to test the anti-PD-L1 antibody durvalumab with or without the CTLA-4 inhibitor tremelimumab in addition to etoposide-platinum chemotherapy (EP) as first-line treatment in patients with extensive-stage small-cell lung cancer (ES-SCLC). Compared to EP only, this three-arm study revealed a significant benefit of durvalumab plus EP regarding overall survival (OS) that was sustained after more than 3 years of follow-up (12.9 vs. 10.5 months; HR, 0.71; p = 0.0003) [1, 2]. Durvalumab plus tremelimumab in addition to EP led to numerical OS improvement vs. chemotherapy alone, with 36-month OS rates of 15.3 % vs. 5.8 % (HR, 0.81) [2, 3]. Considering the lack of well-characterized biomarkers that predict the efficacy of immune checkpoint inhibitors, Paz-Ares et al. presented an analysis of long-term survivors (LTS) at ELCC 2022 [4]. Characteristics were assessed in patients who were still alive at the 22 March 2021 data cut-off after a median follow-up for OS of 39.4 months.
The analysis showed that the CASPIAN trial population contained more than 3 times as many LTS in the durvalumab plus EP arm than in the EP arm (16 % vs. 5 %). Those in the durvalumab/tremelimumab plus EP arm were almost 3 times as many (14 %). Overall, 81 patients in the two immunotherapy arms constituted LTS. At the data cut-off, 46 of them were still receiving durvalumab (n = 27) or both checkpoint inhibitors (n = 19) in addition to chemotherapy. In terms of baseline characteristics, LTS, as compared to the ITT populations, had a higher incidence of favorable prognostic markers such as female gender and good performance status. Although the proportions of patients with brain and liver metastases were lower than in the ITT arms, they were not zero, indicating that some patients achieved long-term survival despite the presence of these lesions.
Clinical and molecular differences
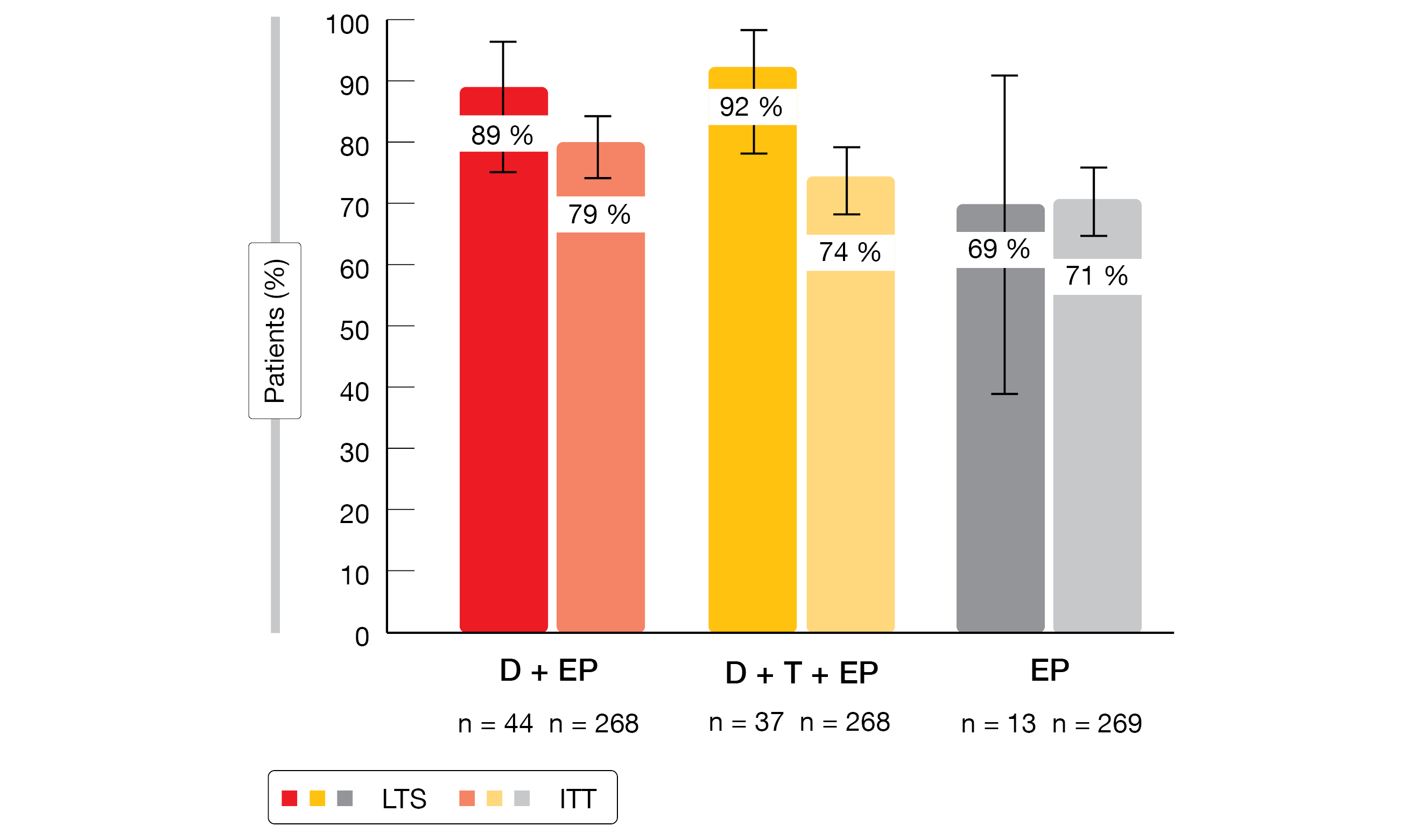
LTS in both checkpoint-inhibitor–based treatment arms were more likely to have completed EP induction and to have achieved objective responses than the ITT population (Figure). Their PFS rates at 12 and 24 months were markedly higher across all arms. No evidence of cumulative toxicity emerged despite longer exposure in LTS, who did not experience increases in serious adverse events (AEs). The distribution of serious AEs across system organ classes was similar for LTS and ITT patients.
With respect to molecular characteristics, the investigators assessed PD-L1 expression, tissue tumor mutational burden (tTMB), and the presence of the HLA-DQB1*03:01 allele. For the durvalumab plus EP arm, the molecular assessments yielded no association of any of these markers with OS ≥ 18 or ≥ 36 months. At the same time, PD-L1 expression ≥ 1 % and the presence of the HLA-DQB1*03:01 allele were enriched in the durvalumab/tremelimumab plus chemotherapy arm in patients with median OS ≥ 18 months vs. those with OS < 18 months. This held true even after 36 months, although the patient numbers grew small over time. The authors noted that further investigation is warranted to understand the potential role of these and other biomarkers in SCLC.
Figure: Objective response rates in long-term survivors (LTS) and the ITT population with durvalumab plus EP (D+EP), durvalumab/tremelimumab plus EP (D+T+EP), and EP alone
Consolidative radiotherapy
The safety and efficacy of thoracic consolidative radiotherapy during immunotherapy in the setting of ES-SCLC has not been reported to date. Daher et al. therefore performed a multicenter, retrospective study that investigated consolidative radiotherapy (defined as radiation given at the end of chemotherapy to responders) in consecutive patients with ES-SCLC who were treated with platinum-based chemotherapy plus durvalumab or atezolizumab [5]. Twenty-five individuals treated at 4 centers in Israel were compared to a group of 101 patients who did not receive consolidative radiotherapy.
Indeed, consolidative radiotherapy was shown to be safe and feasible for patients with ES-SCLC undergoing chemoimmunotherapy. The rates of immune-related AEs were similar across the irradiated and not irradiated groups (12.0 % vs. 14.9 %). No pneumonitis cases were reported as related to consolidative radiotherapy. Grade 3 AEs occurred in 20 % vs. 14.9 %, and no grade 4 or 5 events were identified. In addition, patients receiving consolidative radiotherapy showed longer median PFS (8.5 vs. 5.6 months; HR, 0.48; p < 0.003) and OS (27.7 vs. 13.2 months; HR 0.33; p < 0.007). Prospective studies are required to assess the potential role of consolidative radiotherapy in the treatment of ES-SCLC.
Trial in progress: AdvanTIG-204
In the setting of limited-stage SCLC, no novel therapeutic agents improving clinical outcomes beyond concurrent chemoradiotherapy (cCRT), which represents the standard of care, have been established to date. The randomized, multicenter, open-label, phase II AdvanTIG-204 study is assessing first-line treatment with the anti-TIGIT antibody ociperlimab 900 mg Q3W in addition to the PD-1 inhibitor tislelizumab 200 mg Q3W plus cCRT (Arm A) vs. tislelizumab 200 mg Q3W plus cCRT (Arm B) and cCRT alone (Arm C) [6]. After 4 cycles of treatment, patients in Arms A and B go on to receive ociperlimab plus tislelizumab and tislelizumab monotherapy, respectively.
TIGIT is a co-inhibitory immune checkpoint receptor that is upregulated on T cells and natural killer cells in multiple solid tumors, giving rise to escape from immune surveillance [7, 8]. Dual targeting of tumors with anti-TIGIT and anti-PD-1 monoclonal antibodies has shown synergistic immune activation and enhanced antitumor activity in the phase I AdvanTIG-105 trial [9]. Approximately 120 patients with untreated limited-stage SCLC will be included in AdvanTIG-204. Progression-free survival in the ITT analysis set is defined as the primary endpoint. The first patient was enrolled in July 2021, and the study is ongoing.
REFERENCES
- Paz-Ares L et al., Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019; 394(10212): 1929-1939
- Paz-Ares L et al., Durvalumab ± tremelimumab + platinum-etoposide in 1L extensive-stage SCLC: 3-year overall survival update from the phase III CASPIAN study. Ann Oncol 2021; 32 (suppl_5): S1283-S1346
- Goldman JW et al., Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021; 22(1): 51-65
- Reinmuth N et al., Durvalumab ± tremelimumab + platinum-etoposide in 1L extensive-stage SCLC: characteristics of long-term survivors in the CASPIAN study. ELCC 2022, abstract 141O
- Daher S et al., Real-world data of consolidative radiotherapy for extensive-stage SCLC treated by chemo-immunotherapy. ELCC 2022, abstract 144P
- Lu Y et al., AdvanTIG-204: Anti-TIGIT monoclonal antibody ociperlimab plus anti-PD-1 monoclonal antibody tislelizumab plus concurrent chemoradiotherapy in patients with untreated limited-stage small cell lung cancer. ELCC 2022, abstract 154TiP
- Manieri NA et al., TIGIT: a key inhibitor for the cancer immunity cycle. Trends Immunol 2017; 38(1): 20-28
- Harjunpää H and Guillerey C, TIGIT as an emerging immune checkpoint. Clin Exp Immunol 2020; 200(2): 108-119
- Frentzas S et al., AdvanTIG-105: Phase 1 dose-escalation study of anti-TIGIT monoclonal antibody ociperlimab (BGB-A1217) in combination with tislelizumab in patients with advanced solid tumors. J Clin Oncol 2021; 39 (15_suppl): 2583
© 2022 Springer-Verlag GmbH, Impressum