Clinical trial endpoint selection
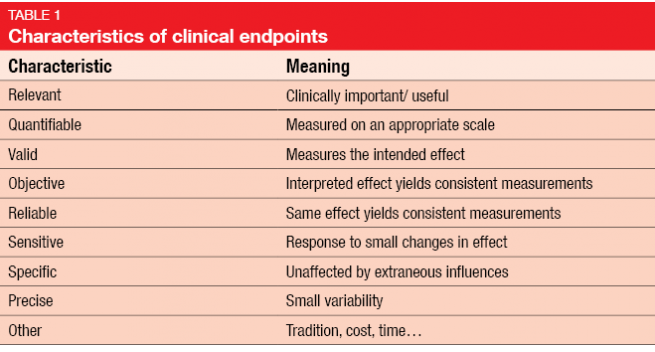
Endpoint selection is a crucial aspect in the context of clinical trial design. The investigated outcomes should be clinically relevant, reliable, sensitive and specific, among others (Table 7). Legal requirements characterize the primary endpoint as a valid and reliable measure that provides the most clinically relevant and convincing evidence.
Definitions
Commonly used endpoints are based on survival, tumor response, and symptom assessment. Historically, overall survival (OS) has been viewed as the most effective measure as it addresses the biology of the tumor and the natural history of disease. PFS possesses significance because it assesses tumor shrinkage and stabilization of disease. Response rates enable objective demonstration of the drug effect; also, durability of response is taken into consideration. From the patient point of view, symptom assessment is one of the most important endpoints.
Phase III or confirmatory studies call for OS as the primary endpoint. PFS is possible where clinically relevant and can be regarded as a surrogate of OS when OS differences cannot be achieved due to crossover. RR is not recommended as the primary endpoint in this setting. For accelerated approval, the results must predict the clinical benefit of the new drug/ combination over the available therapy. Here, overall RR and response duration have been the most commonly used surrogate endpoints. PFS is also acceptable under some conditions.
In the phase III setting, the absolute magnitude of the treatment benefit needs to be taken into account when judging the activity of a certain regimen. Large numbers of recruited patients can render the differences between the study drug and the comparator statistically significant, but this does not automatically imply clinical significance. However, randomized controlled trials only require large sample sizes when the goal is the identification of small treatment effects.
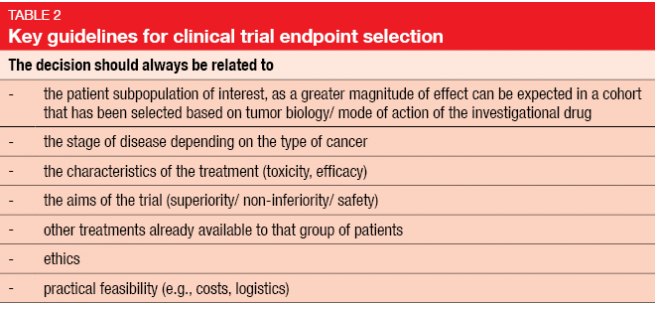
Generally, the selected endpoints should be reflective of patient benefit; therefore, it is commendable to use OS, PFS and some assessment of symptom relief or quality of life. Table 2 specifies key guidelines for clinical trial endpoint selection.
Overall survival
OS is accepted as the gold standard for the evaluation of oncological agents. It is objective, easily measureable, clinically significant, accurate, not prone to investigator or assessment bias, and readily comparable across diseases.8 If OS is used as a secondary endpoint, the trial should be powered sufficiently for the assessment of this outcome.
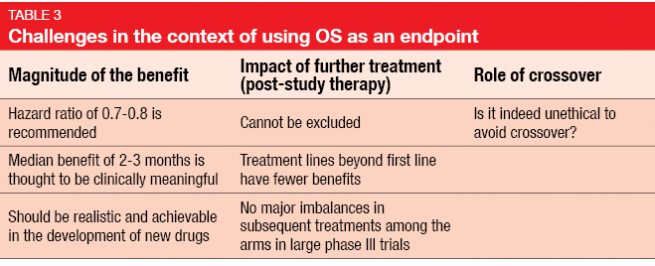
Several drawbacks of OS prevent its use in many trials, however. OS requires long observational periods and large sample sizes; it is influenced by post-trial therapy and complex to analyze when many salvage agents have been administered after completion of the study. Overall, this endpoint makes trial conduct comparatively expensive. Challenges on the statistical level comprise the magnitude of the benefit, the impact of further treatment, and the role of crossover (Table 3). Hazard ratios of 0.7–0.8 are recommended, and a median OS benefit of 2–3 months is thought to be clinically meaningful.
According to the ASCO Consensus, which emphasizes the use of OS as an endpoint, clinical trials should aim to improve OS by at least 25 % [1]. The Consensus states that agents not expected to provide such an OS gain would no longer be needed in clinical practice. However, this threshold was arbitrarily defined and is rather difficult to achieve for many large phase III clinical trials. The same guideline refers to exceptions in certain clinical situations, such as the emergence of secondary driver mutations after progression during first-line targeted therapies. Success can be obtained with second-line treatment in these patients, but this makes OS difficult to assess. The use of PFS as a clinically meaningful endpoint can be appropriate here. PFS has been employed for the clinical assessment of many targeted drugs approved in non-small-cell lung cancer. However, OS continues to be an important endpoint, as for example the proof of OS gains through the addition of particular drugs to chemotherapy can be indispensable.
Progression-free survival
Surrogate markers offer several advantages. They can be measured earlier, more frequently and in a more convenient or less invasive way than established markers. Also, reductions in the size of clinical trials and shortening of trial duration are rendered possible, which results in acceleration of the approval process. PFS is widely used as a surrogate endpoint for OS in trials on targeted agents. Compared to OS, it is less influenced by competing causes of death or post-progression therapy. Progression events occur early and more frequently than death events, which optimizes assessment. The oncological community harbors the growing belief that delaying progression in metastatic disease is worthwile, even if OS is not improved. From the patient point of view, PFS has become an important outcome due to the psychological significance of disease progression and its implications for quality of life. Accordingly, the number of studies using PFS as an endpoint has increased by a massive amount over the last decades [2].
On the other hand, PFS can be affected by assessment bias (investigators declare patients in the control arm progressors to get them on the active experimental therapy as soon as possible), evolution-time bias (suspected progression may be formally evaluated later in one arm than in the other), and attrition bias (more patients withdraw from one arm than from the other). Also, the exact time of progression tends to be unknown, as this depends on the frequency of radiological assessment. If imaging is performed every 3 months, a given patient can have been progressive for 2 months already at the time of the next assessment after he had been declared progression-free at the last one. Moreover, clinical progression (e.g., weight loss, increases in symptom burden) without any visible increases in tumor size can occur. Assessment bias is the reason why independent review has become a certain standard to verify measurements.
Overall, PFS is challenging to employ as a regulatory endpoint, but it will continue to have a future role in oncology drug registration if rigorous acceptance criteria and standards are met. There will be increasing regulatory pressure to link or associate PFS benefits with other clinical trial outcomes that show direct clinical benefit (e.g. quality of life benefits, disease-related symptom benefits, positive OS trends). PFS might have its best future applications in symptomatic disease and/ or in settings where delay in disease progression correlates with delay in symptom onset.
REFERENCES
- http://www.asco.org/practice-research/clinically-meaningful-outcomes
- Booth CM, Eisenhauer EA, Progression-free survival: meaningful or simply measurable? J Clin Oncol 2012; 30(10): 1030-1033
Author: James Yang
© 2017 Springer-Verlag GmbH, Impressum