Cold agglutinin disease: on the road to new insights and potential treatment options
Cold agglutinin disease (CAD) is a rare type of autoimmune hemolytic anemia (AIHA) elicited by cold-sensitive antibodies including cold agglutinins. Ninety percent of cold agglutinins belong to the IgM kappa category and bind to red blood cell surface antigens at temperatures of ≤ 37 °C, thus inducing hemolysis [1-3]. CAD accounts for approximately 25 % of AIHA cases, with an incidence and prevalence of 1 case per million persons per year and 16 cases per million persons, respectively [4, 5]. The primary type of this disease is a chronic condition usually associated with low-grade lymphoproliferation and is typically found in older adults (median age of onset, 67 years) [4, 5]. Secondary CAD is termed cold agglutinin syndrome (CAS) and arises based on underlying conditions such as malignancies or acute infections [5, 6]. An associated disorder is mixed warm and cold antibody AIHA [5].
Patients affected by CAD show a high burden of disease. IgM-antigen complexes activate complement-dependent extravascular and (to a lesser degree) intravascular hemolysis, which leads to anemia and debilitating fatigue [7, 8]. The risk of thromboembolism is increased, and the 5-year mortality is higher than in matched controls [9, 10]. Approved treatments for CAD are still lacking. Current approaches such as B-cell–directed therapies and chemotherapies elicit only poor response rates and can give rise to substantial toxicity [6, 11].
The CADENCE Registry
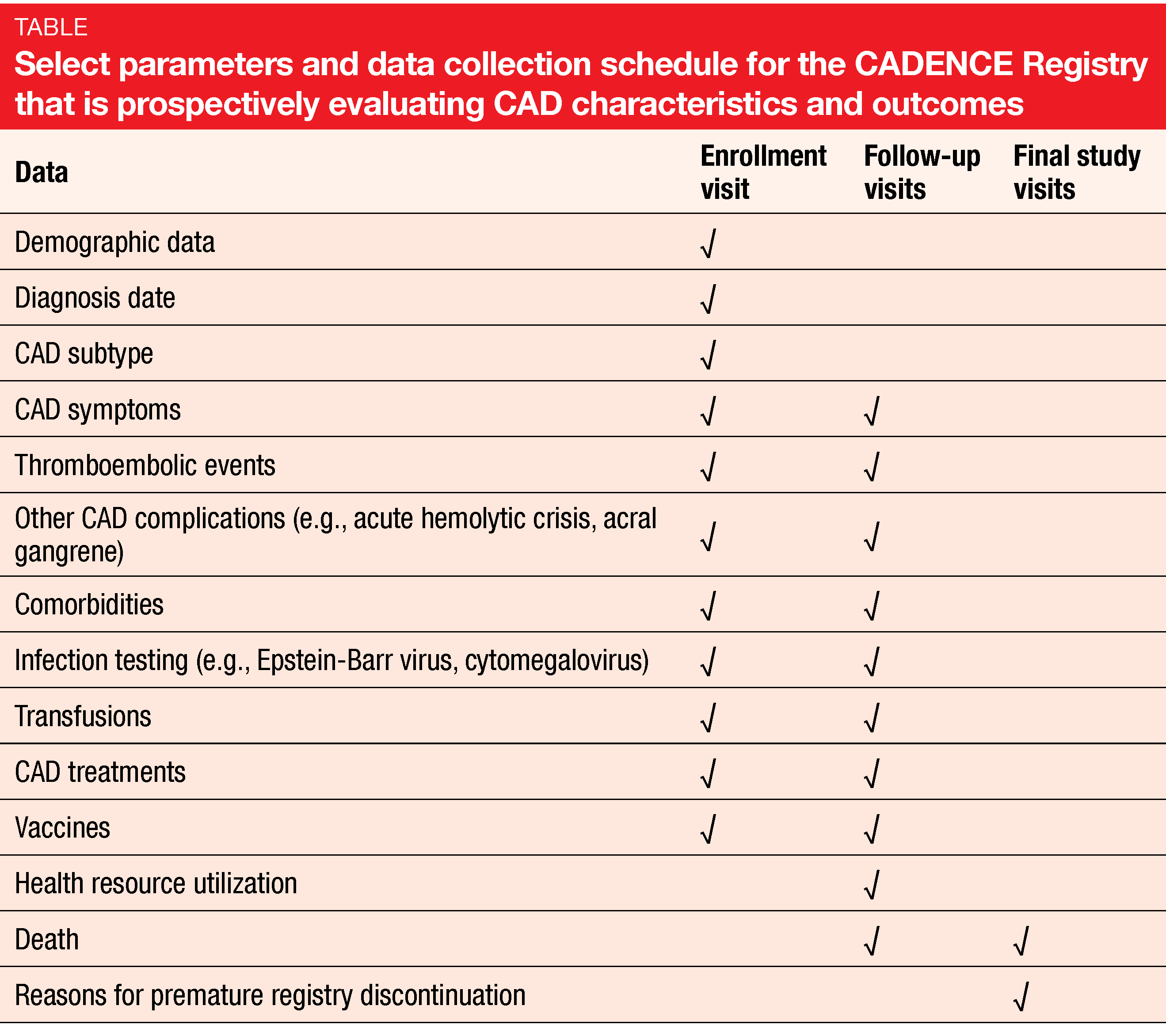
Given the rarity of CAD, there is a paucity of prospective longitudinal data describing patient and clinical characteristics as well as outcomes. This gap will be filled by the observational, non-interventional, multicenter, prospective, longitudinal CADENCE Registry that was launched in December 2019 [12]. Data from more than 700 adults ≥ 18 years of age with CAD, CAS, or mixed warm and cold antibody AIHA are being collected at 121 sites in 11 countries across the globe including the US, France, UK, Germany, Austria, Japan and Australia. The recruitment period will end in late 2021, and patients will be followed until late 2024.
Objectives of this registry are to better understand patient and clinical characteristics, patterns and use of CAD treatments, long-term clinical outcomes, patient health-related quality of life, and healthcare resource utilization (Table). Also, the natural history of CAD including complications and comorbidities will be explored. Interim analyses will be conducted after the enrollment of 100, 250, and 500 patients.
Quality of life data from the Cardinal study
The first-in-class humanized monoclonal anti-C1s antibody sutimlimab is being investigated for the treatment of patients with CAD. By inhibiting the serine protease C1s of the C1 complex, sutimlimab blocks complement-mediated tissue damage and prevents the long-term activation of autoimmune B cells, as well as the production of autoantibodies [13]. The open-label, single-arm, multicenter, phase III Cardinal study evaluated the efficacy and safety of sutimlimab in CAD patients with baseline hemoglobin levels ≤ 10 g/dL and active hemolysis (i.e., total bilirubin > normal) who had received at least one blood transfusion within 6 months of enrollment. Part A of the trial assessed the efficacy and safety of sutimlimab 6.5 g (body weight < 75 kg) or 7.5 g (≥ 75 kg) intravenously on days 0 and 7 followed by the same doses every 2 weeks for a total of 26 weeks. Part B is an ongoing, long-term extension study.
Health-related quality-of-life outcomes were secondary, exploratory endpoints of the trial. Röth et al. reported the results for this outcome at the EHA Congress 2020 [14]. Measurements included the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale, the 12-Item Short Form Health Survey (SF-12), the EuroQoL (EQ)-5D Index and visual analog scale scores, the Patient Global Impression of Change (PGIC) scale, and the Patient Global Impression of (Fatigue) Severity (PGIS) scale. The patients (n = 24) had a mean age of 71 years and were mainly female (62.5 %). Over the previous 6 months, they had received a mean of 3.2 transfusions. Within the last 5 years, the majority had been treated with at least one targeted CAD therapy. One third had a history of at least one thromboembolic event.
Rapid and durable improvements
Treatment with sutimlimab was shown to give rise to rapid, clinically meaningful improvements in all patient-reported outcome measures evaluated. Almost 90 % of patients achieved clinically meaningful improvement (≥ 3-point increase) of the FACIT-F score. This change occurred already within a week from the initiation of treatment and was associated with an inhibition of the classical complement pathway as measured by the Wieslab-CP assay and assessment of the C4 levels. Likewise, improvement in the SF-12 scores correlated with near-complete inhibition of the complement pathway and normalization of C4. These observations suggest that in addition to anemia, pathway activation with subsequent hemolysis is a key driver of fatigue and poor quality of life in patients with CAD.
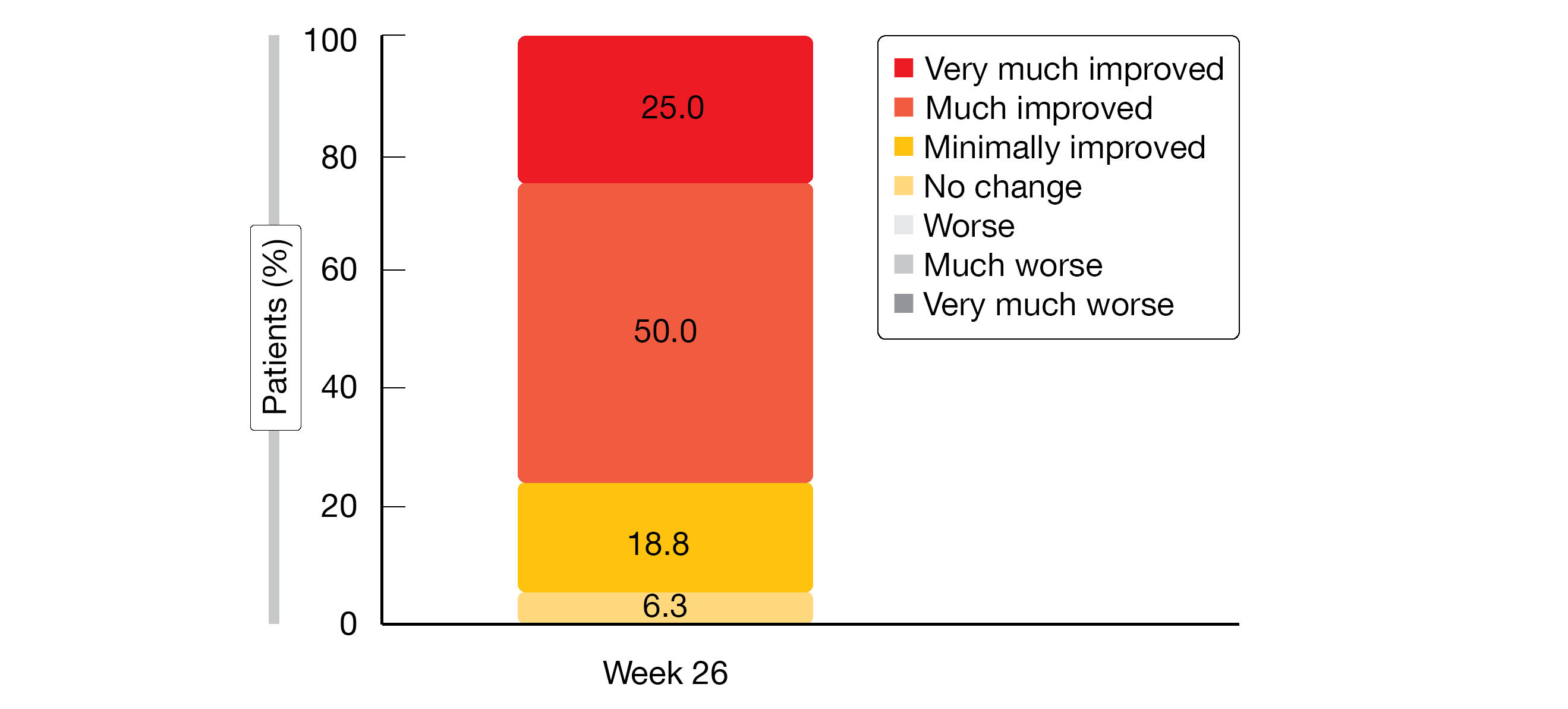
Clinically meaningful increases in both the SF-12 physical and mental component scores were observed at week 5 and proved durable. The EQ-5D index and EQ-5D VAS scores increased by 0.074 and 16.8, respectively, until week 26. Improvements were observed for each of the EQ-5D domains; moderate, severe or extreme problems with regard to mobility, self-care, usual activities, pain/discomfort and anxiety/depression decreased in the course of the study. With respect to PGIC, 93.8 % of patients observed global improvement until week 26 (Figure). Seventy-five percent noted that their condition had improved much or very much.
Fatigue was mild or moderate in 88.2 % of patients according to PGIS at week 26, with the remaining 11.8 % indicating no change. At baseline, a total of 83.3 % of patients had reported fatigue that had been severe in a third of cases. No patient experienced worsening of their general condition or severe fatigue at the end of treatment. Overall, these outcomes further support the efficacy of targeting the classical complement pathway in the management of patients with CAD.
Figure: Patient Global Impression of Change (PGIC): patient-reported results at the end of the 26-week sutimlimab treatment period
REFERENCES
- Berentsen S et al., Primary chronic cold agglutinin disease: an update on pathogenesis, clinical features and therapy. Hematology 2007; 12(5): 361-370
- Berentsen S et al., Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am 2015; 29(3): 455-471
- Gertz MA, Management of cold haemolytic syndrome. Br J Haematol 2007; 138(4): 422-429
- Berentsen S et al., Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica 2006; 91(4): 460-466
- Berentsen S et al., Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Reviews 2012; 26(3): 107-115
- Jäger U et al., Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev 2020; 41: 100648
- Berentsen S, Sundic T, Red blood cell destruction in autoimmune hemolytic anemia: role of complement and potential new targets for therapy. Biomed Res Int 2015; 2015: 363278
- Swiecicki PL et al., Cold agglutinin disease. Blood 2013; 122(7): 1114-1121
- Broome CM et al., Increased risk of thrombotic events in cold agglutinin disease: a 10-year retrospective analysis. Res Pract Thromb Haemost 2020; 4(4): 628-635
- Bylsma LC et al., Occurrence, thromboembolic risk, and mortality in Danish patients with cold agglutinin disease. Blood Adv 2019; 3(20): 2980-2985
- Berentsen S, How I manage patients with cold agglutinin disease. Br J Haematol 2018; 181(3): 320-330
- Röth A et al., Cold Agglutinin Disease Real World Evidence (CADENCE) Registry: design of the first international, prospective CAD Registry. EHA 2020, abstract EP1618
- Nikitin PA et al., C1s inhibition by BIVV009 (sutimlimab) prevents complement-enhanced activation of autoimmune human B cells in vitro. J Immunol 2019; 202(4): 1200-1209
- Röth A et al., Sutimlimab, a complement C1s inhibitor, improves quality of life in patients with cold agglutinin disease: patient-reported outcomes results of the phase 3 Cardinal study. EHA 2020, abstract S333
More posts
Waldenström’s macroglobulinemia: BTK inhibition and other treatments
Waldenström’s macroglobulinemia: BTK inhibition and other treatments Within the
Preface – EHA 2020
Preface – EHA 2020 Constantine Tam, MB, BS (Hons), MD, FRACP, FRCPA, Peter MacC