Marginal zone lymphoma: benefits of BTK inhibition in later lines
Zanubrutinib in patients ≥ 65 years
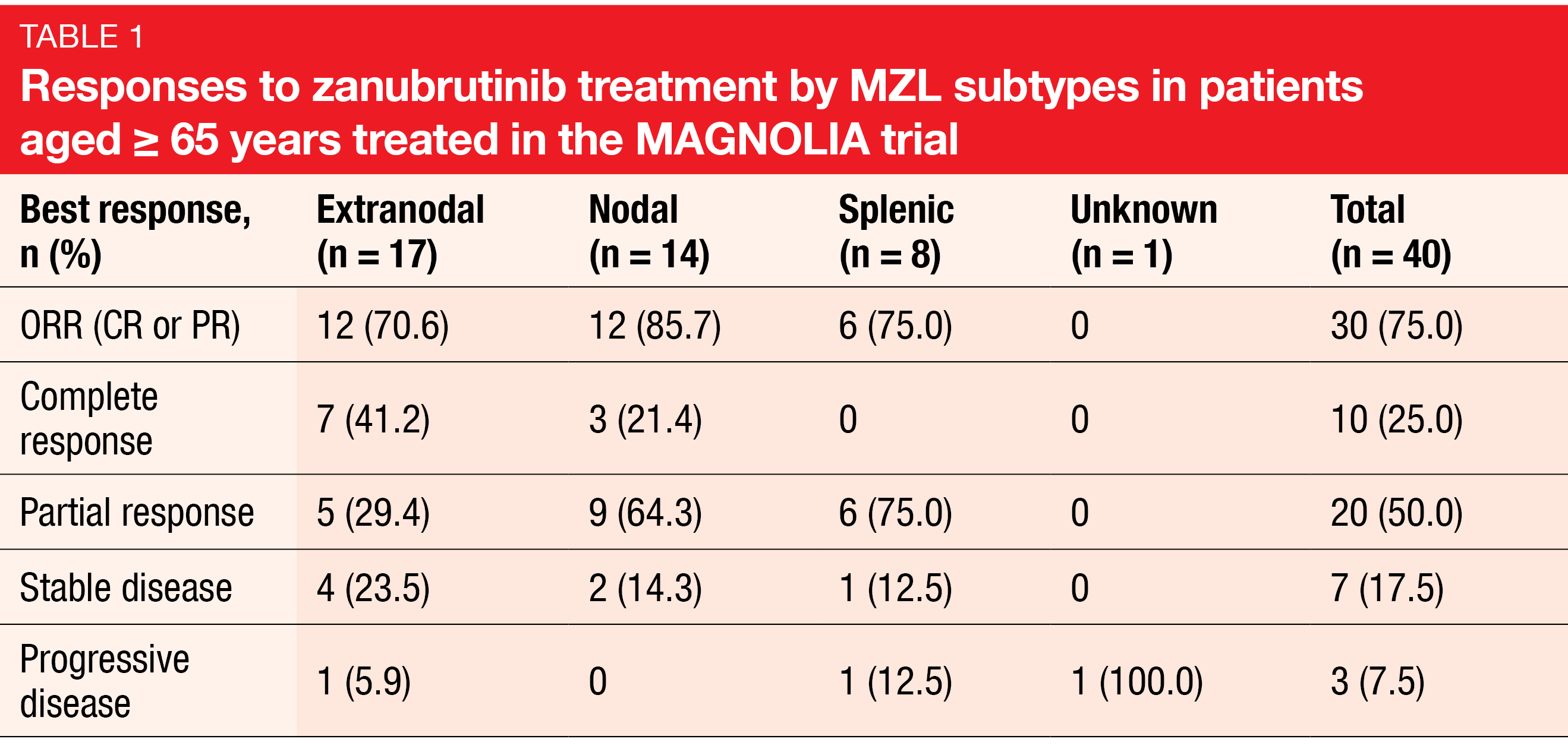
Patients with marginal zone lymphoma (MZL) usually show an indolent course of disease, although MZL remains largely incurable, particularly in the relapsed/refractory setting [1, 2]. BTK inhibition offers a potent treatment option in this situation. The single-arm, multicenter, phase II MAGNOLIA study tested the next-generation BTK inhibitor zanubrutinib in patients with relapsed/refractory MZL who had received ≥ 1 CD20-based regimen, demonstrating high response rates and durable disease control [3]. As MZL is the second most common lymphoma in older patients, patient- or disease-related risk factors and treatment-related toxicities can make the selection of an optimal treatment challenging [4]. Opat et al. investigated zanubrutinib in the subgroup of patients aged ≥ 65 years included in the MAGNOLIA trial (n = 40) [5].
Zanubrutinib proved well tolerated and highly effective in the older population. In the group aged ≥ 65 years, the overall response rate (ORR) was 75 %, and in those aged ≥ 75 years (n =18), 94.4 %. Complete remissions resulted in 25 % and 22.2 %, respectively. Responses were observed across all subgroups independent of line of treatment, time since the last anti-lymphoma therapy, disease status (relapsed vs. refractory), the presence of bulky disease, the type of prior treatment, and MZL subtype (Table 1). Median progression-free survival (PFS) and duration of response had not been reached yet. At 15 months, 86.6 % of patients were alive and progression-free.
Treatment-emergent AEs were mostly grade 1 and 2, and none gave rise to dose reductions. Events leading to dose interruption and study drug discontinuation were reported in 32.5 % and 5.0 %, respectively. Atrial flutter/fibrillation and hypertension occurred in 2 cases each but did not lead to treatment withdrawal. No major hemorrhage was observed. At a median study follow-up of 15.8 months, 87.5 % of patients were still on treatment. In their summary, the authors pointed out that these results are consistent with previously published findings [3].
Proof-of-concept study for acalabrutinib
A multicenter, open-label, phase II study examined monotherapy with the next-generation BTK inhibitor acalabrutinib in patients with relapsed/refractory MZL after ≥ 1 systemic treatment line containing ≥ 1 CD-20–directed regimen. ORR as assessed by the investigator constituted the primary endpoint. At ASCO 2022, Budde et al. presented an interim report of the trial after a median follow-up of 13.3 months [6].
Among 40 evaluable patients, the ORR was 52.5 %, including complete and partial responses in 13 % and 40 %, respectively. The subgroup with the extranodal subtype fared best (ORR, 64.7 %); ORRs for those with nodal and splenic MZL were 41.7 % and 45.5 %, respectively. Ninety-three percent of patients experienced tumor reductions. Median duration of response had not been reached yet, which also applied to median overall survival. Median PFS was 27.4 months.
The AEs reported were consistent with the known safety profile of acalabrutinib. Most events were rated as grade 1 or 2. The most common grade ≥ 3 AEs included fatigue, neutropenia, anemia, dyspnea, and thrombocytopenia. Among events of special clinical interest, infections (34.9 %) and bleeding (23.3 %) were most common. AEs led to acalabrutinib discontinuation in 7 %. One patient died due to an AE, which was septic shock occurring after approximately 5.2 months of acalabrutinib treatment. No cases of atrial fibrillation/flutter, ventricular arrhythmias, or major hemorrhage were reported. Overall, the results of this study support acalabrutinib as a safe and feasible chemotherapy-free option.
Economic burden of MZL
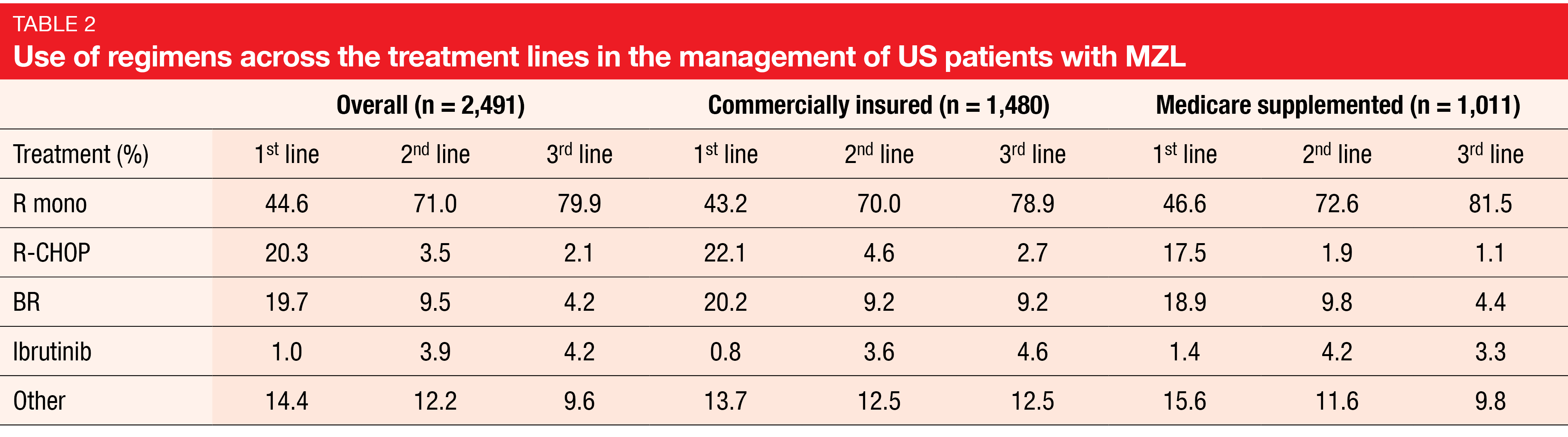
Based on the IBM MarketScan® commercial and Medicare supplemental claims dataset, a retrospective, observational study assessed real-world treatment patterns, costs, and healthcare resource utilization in US patients with MZL between 2017 and 2020 [7]. Overall, 2,491 patients with a median age of 63 years were identified. Among these, 59 % were commercially insured (median age, 57), while 41 % received Medicare-supplemented care (median age, 76).
The findings suggested that real-world treatment patterns in the US across lines of therapy follow the regimen recommendations by the National Comprehensive Cancer Network clinical practice guidelines. First-line, second-line and third-line treatment was administered in 72 %, 29 %, and 13 %, respectively. Single-agent rituximab represented the most common regimen across both commercial and Medicare patients and all treatment lines (Table 2). R-CHOP and bendamustine/rituximab were the second most commonly used regimens in first line with decreased use in later lines, where ibrutinib showed increasing use although it had lower per person per month (PPMM) cost in the first-line setting than other treatments.
MZL patients were demonstrated to incur a high economic burden. Overall, they had 4.6 outpatient visits PPMM, as well as 0.5 hospitalizations with a mean length of stay of 2.6 days. The total PPMM healthcare costs amounted to $ 19,896. Multivariable regression showed that baseline comorbidities such as atrial fibrillation, renal disease and neutropenia, as well as treatment discontinuation, were significant predictors of higher costs and healthcare resource utilization. According to the authors, future studies are needed to evaluate long-term outcomes and the impact of heterogenous MZL subtypes.
REFERENCES
- Cheah CY et al., Marginal zone lymphoma: present status and future perspectives. Haematologica 2022; 107(1): 35-43
- National Cancer Institute 2020 https://seer.cancer.gov/archive/csr/1975_2017/, accessed April 2022
- Opat S et al., The MAGNOLIA trial: zanubrutinib, a next-generation Bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res 2021; 27(23): 6323-6332
- Bron D et al., Marginal zone lymphomas: second most common lymphomas in older patients. Curr Opin Oncol 2019; 31(5): 386-393
- Opat S et al., Zanubrutinib in older patients with relapsed/refractory marginal zone lymphoma: subgroup analysis of the MAGNOLIA study. EHA 2022, P1162
- Budde LE et al., Acalabrutinib in patients with relapsed/refractory marginal zone lymphoma: results of a phase 2, multicenter, open-label trial. J Clin Oncol 40, 2022 (suppl 16; abstr 7549)
- Yang K et al., Real-world treatment patterns and economic burden of patients with marginal zone lymphoma. J Clin Oncol 40, 2022 (suppl 16; abstr e18730)
© 2022 Springer-Verlag GmbH, Impressum