New options in untreated and pretreated mantle cell lymphoma
SHINE trial: first-line regimen for older patients
Patients of an advanced age with previously untreated mantle cell lymphoma (MCL) usually receive chemoimmunotherapy regimens such as bendamustine/rituximab (BR), R-CHOP or bortezomib/rituximab/cyclophosphamide/doxorubicin/prednisone (VR-CAP), with BR having become the most commonly used first-line strategy [1]. Two independent observational studies have shown significantly improved progression-free survival (PFS) for BR followed by rituximab maintenance [1, 2]. Also, ibrutinib, which has transformed the treatment of patients with relapsed/refractory MCL, has been tested successfully in the first-line phase IB setting in combination with BR [3].
Based on this information, the randomized, double-blind, phase III SHINE study was designed to assess ibrutinib until disease progression in addition to BR induction for 6 cycles in patients with previously untreated, stage II-IV MCL who were ≥ 65 years of age. Those who obtained complete or partial response after BR induction went on to receive rituximab maintenance every 8 weeks for 12 cycles. The control arm was treated with BR induction and rituximab maintenance plus placebo. At 183 study sites in 28 countries, a total of 523 patients were randomized in a 1:1 manner. The PFS in the ITT population constituted the primary endpoint.
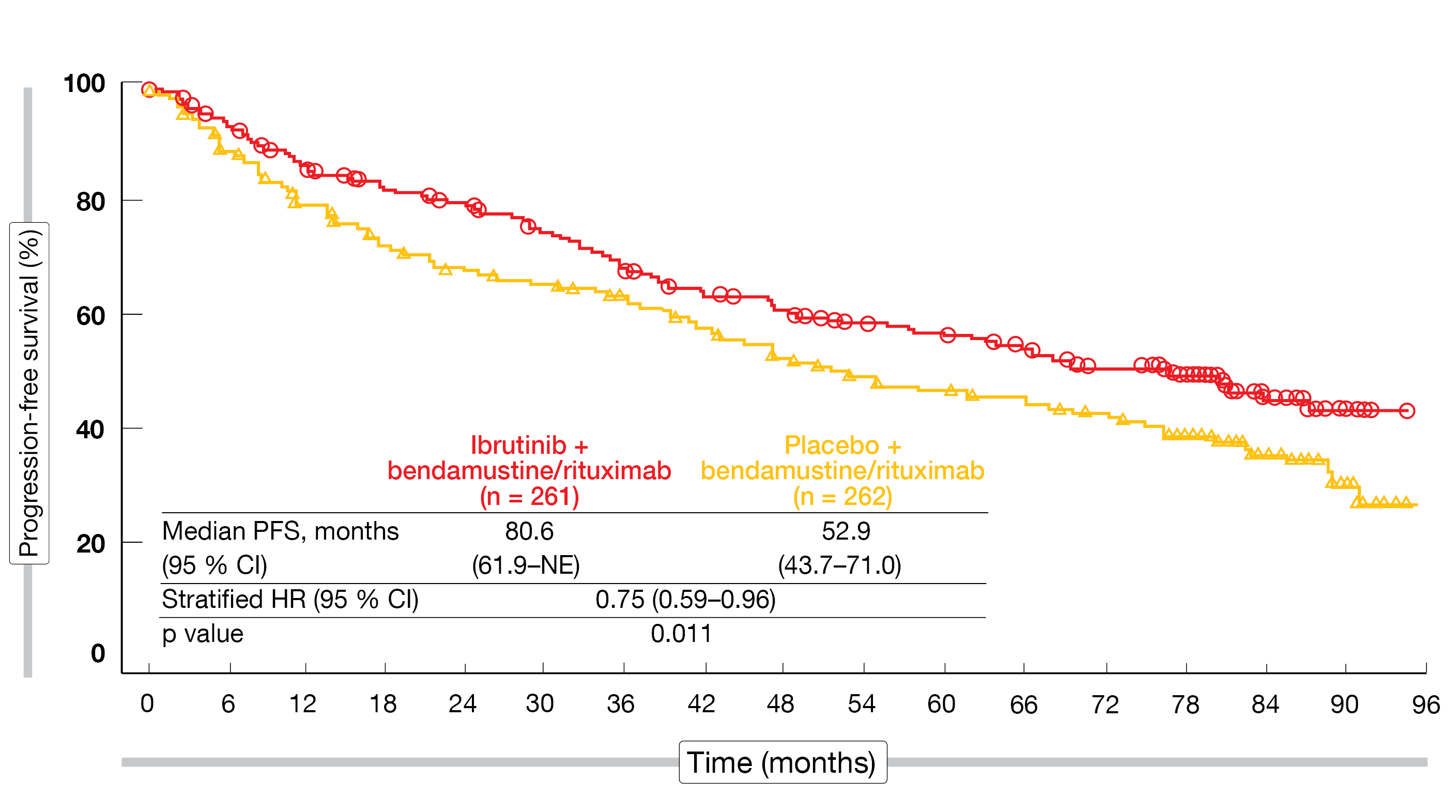
SHINE was the first phase III study to show that ibrutinib plus chemoimmunotherapy is highly effective in patients with newly diagnosed MCL. According to the results presented at EHA 2022 by Wang et al., the primary endpoint was met after a median follow-up of 84.7 months, with a statistically significant and clinically meaningful 2.3-year advantage and a 25 % reduction in the risk of progression or death (80.6 vs. 52.9 months; HR, 0.75; p = 0.011; Figure) [4]. The PFS benefit emerged early on in the first year and persisted over time. Almost all subgroups treated with ibrutinib plus chemoimmunotherapy benefited to a greater extent in terms of PFS. However, no advantage was noted in the group with high risk according to the Mantle Cell Lymphoma International Prognostic Index (MIPI).
Figure: Improved progression-free survival with ibrutinib plus chemoimmunotherapy vs. placebo plus chemoimmunotherapy in the SHINE study
Additional outcomes
The overall response rates (ORRs) were comparable across the arms (89.7 % vs. 88.5 %), although complete remissions occurred numerically more frequently with the ibrutinib-based regimen (65.5 % vs. 57.6 %; p = 0.057). Time to next treatment was significantly longer in the experimental arm (not reached vs. 92.0 months; HR, 0.48). Markedly fewer ibrutinib-treated patients required subsequent second-line anti-lymphoma treatment (19.9 % vs. 40.5 %), with 11.5 % vs. 38.7 % receiving BTK inhibition. Median overall survival had not been reached yet in either arm. At 84 months, 55 % vs. 57 % of patients were alive.
The adverse events (AEs) noted in the experimental arm were consistent with the known profiles of ibrutinib and BR. Neutropenia represented the most common treatment-emergent adverse event (TEAE) in the entire study population. Rash, diarrhea, thrombocytopenia, anemia and pneumonia were seen more frequently with the ibrutinib-based treatment. Regarding TEAEs of special clinical interest, any bleeding occurred predominantly in the ibrutinib arm (42.9 % vs. 21.5 %), whereas the rates for major bleeding did not differ (5.8 % vs. 4.2 %). Atrial fibrillation was reported more commonly with ibrutinib plus chemoimmunotherapy (13.9 % vs. 6.5 %), while no differences became apparent for hypertension or arthralgia. Bleeding and atrial fibrillation were generally not treatment-limiting. During the entire study period, second primary malignancies including skin cancers emerged in 21 % vs. 19 %. Death due to TEAEs occurred in 10.7 % vs. 6.1 %. An exploratory analysis of cause-specific survival including only deaths due to disease progression and TEAEs yielded an HR of 0.88 in favor of ibrutinib plus chemoimmunotherapy.
In their summary, the authors emphasized that the median PFS of 6.7 years observed in SHINE is the longest PFS ever published in this population. The study has set a new benchmark for the first-line treatment of older patients with MCL or those unsuitable for autologous stem cell transplantation.
Promising results for pirtobrutinib after BTK inhibition
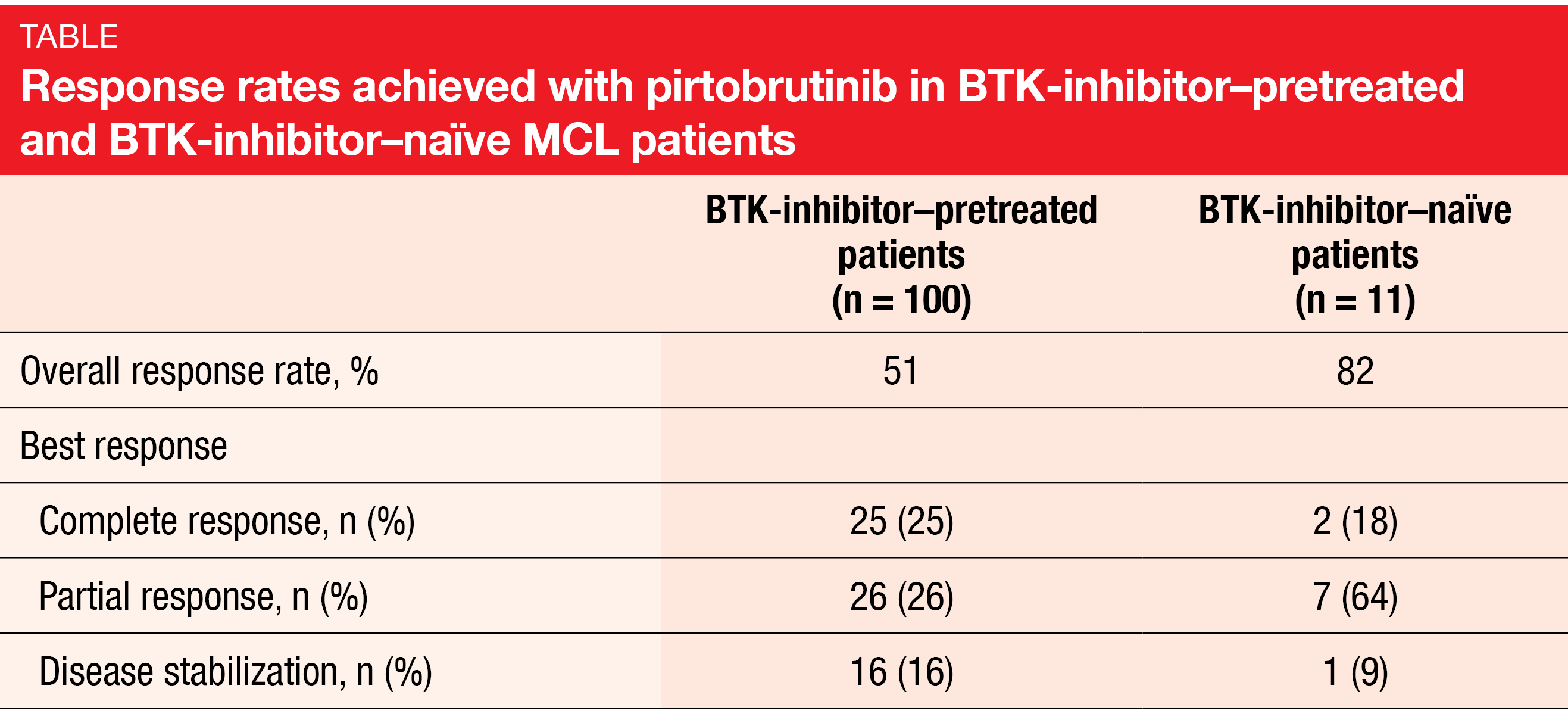
Patients with MCL who progress following treatment with covalent BTK inhibition show poor overall survival [5-7]. The potent and selective non-covalent BTK inhibitor pirtobrutinib is being investigated in patients with MCL in the phase I/II BRUIN study. Eyre et al. reported updated results from the safety population (n = 134) and the efficacy population (n = 111) that included 100 BTK-inhibitor–pretreated and 11 BTK-inhibitor–naïve patients [8].
Overall, 51 % and 82 % of pretreated and naïve patients, respectively, responded to the pirtobrutinib therapy (Table). Complete remissions were obtained in 25 % and 18 %, respectively. Efficacy of the treatment was also seen in patients with prior stem cell transplantation (n = 28; ORR, 64 %) and prior CAR-T therapy (n = 6; ORR, 50 %). After a median follow-up of 8.2 months for responders, 60 % of responses were ongoing.
Moreover, pirtobrutinib showed favorable safety and tolerability, with low rates across the range of side effects. Fatigue was the most common TEAE (23 %), followed by diarrhea (19 %), neutropenia (18 %), and contusion (17 %). Grade 3/4 events were rare. TEAEs of special interest included bruising (22 %), rash (11 %), arthralgia (11 %), hemorrhage (8 %), hypertension (7 %), and atrial fibrillation/flutter (2 %). These were mainly grade 1/2 events. No dose-limited toxicities were reported, and the maximum tolerated dose was not reached. Only 1 % of patients permanently discontinued pirtobrutinib therapy due to treatment-related adverse events.
As the authors noted, pirtobrutinib demonstrated promising efficacy in MCL patients previously treated with BTK inhibitors. The ongoing, randomized, global, phase III BRUIN MCL-321 trial is comparing pirtobrutinib with investigator’s choice of covalent BTK inhibitors in BTK-inhibitor–naïve patients with relapsed MCL (NCT04662255).
REFERENCES
- Martin P et al., Real-world treatment patterns and outcomes of 3,455 previously untreated mantle cell lymphoma patients in U.S. routine clinical practice. J Clin Oncol 2021; 39(suppl 15): 7504
- Hill BT et al., Maintenance rituximab is associated with improved overall survival in mantle cell lymphoma patients responding to induction therapy with bendamustine + rituximab. Hematol Oncol 2019; 37(S2): 405-407
- Maddocks K et al., A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood 2015; 125(2): 242-248
- Wang ML et al., Primary results from the phase 3 SHINE study of ibrutinib in combination with bendamustine-rituximab and R maintenance as a first-line treatment for older patients with mantle cell lymphoma. EHA 2022, S209
- Cheah CY et al., Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 2015; 26(6): 1175-1179
- Martin P et al., Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016; 127(12): 1559-1563
- Rai S et al., MCL-041: Outcomes for recurrent mantle cell lymphoma post-BTK inhibitor therapy in Japan: An administrative database study. Clin Lymphoma Myeloma Leuk 2021; 21(Suppl 1): S407-S408
- Eyre et al., Pirtobrutinib, a highly selective, non-covalent (reversible) BTK inhibitor in previously treated mantle cell lymphoma: updated results from the phase 1/2 BRUIN study. EHA 2022, P1101
© 2022 Springer-Verlag GmbH, Impressum