Prolonging remission in relapsed and refractory follicular lymphoma
Follicular lymphoma (FL) is a common indolent form of non-Hodgkin lymphoma that accounts for 20-25 % of all new NHL cases in Western countries [1]. Although patients with FL generally respond well to first-line anti-CD20-based chemotherapy regimens, recurrence is common. Approved treatment options are limited in the relapsed and refractory setting. Studies suggest that each subsequent relapse involves shorter duration of response [2].
ROSEWOOD: durable responses with zanubrutinib/obinutuzumab
The combination of obinutuzumab with the next-generation BTK inhibitor zanubrutinib was investigated in the phase II, randomized ROSEWOOD trial after phase Ib data had provided an early efficacy signal for this regimen, with good tolerability [3]. Zinzani et al. reported the primary analysis of ROSEWOOD at EHA 2022 after a median follow-up of 12.5 months [4]. The trial included patients with relapsed/refractory grade I-IIIA FL after ≥ 2 lines of therapy including an anti-CD20 antibody and an appropriate alkylator-based combination therapy. While 145 individuals received zanubrutinib plus obinutuzumab, 72 were treated with single-agent obinutuzumab; these were allowed to cross over to the combination in case of confirmed disease progression or lack of response at 12 months.
The study met its primary endpoint, which was overall response superiority of the combination vs. obinutuzumab by independent review (68.3 % vs. 45.8 %; p = 0.0017). Notably, the rate of complete remissions for patients in the experimental arm was nearly double that of patients in the control arm (37.2 % vs. 19.4 %; p = 0.0083). Almost all pre-defined subgroups derived overall response rate (ORR) advantages from zanubrutinib/obinutuzumab vs. obinutuzumab alone. Twenty-nine patients crossed over from the control arm to the experimental arm; in this group, the subsequent ORR amounted to 24.1 % according to investigator assessment, with only 2 patients (6.9 %) obtaining complete response.
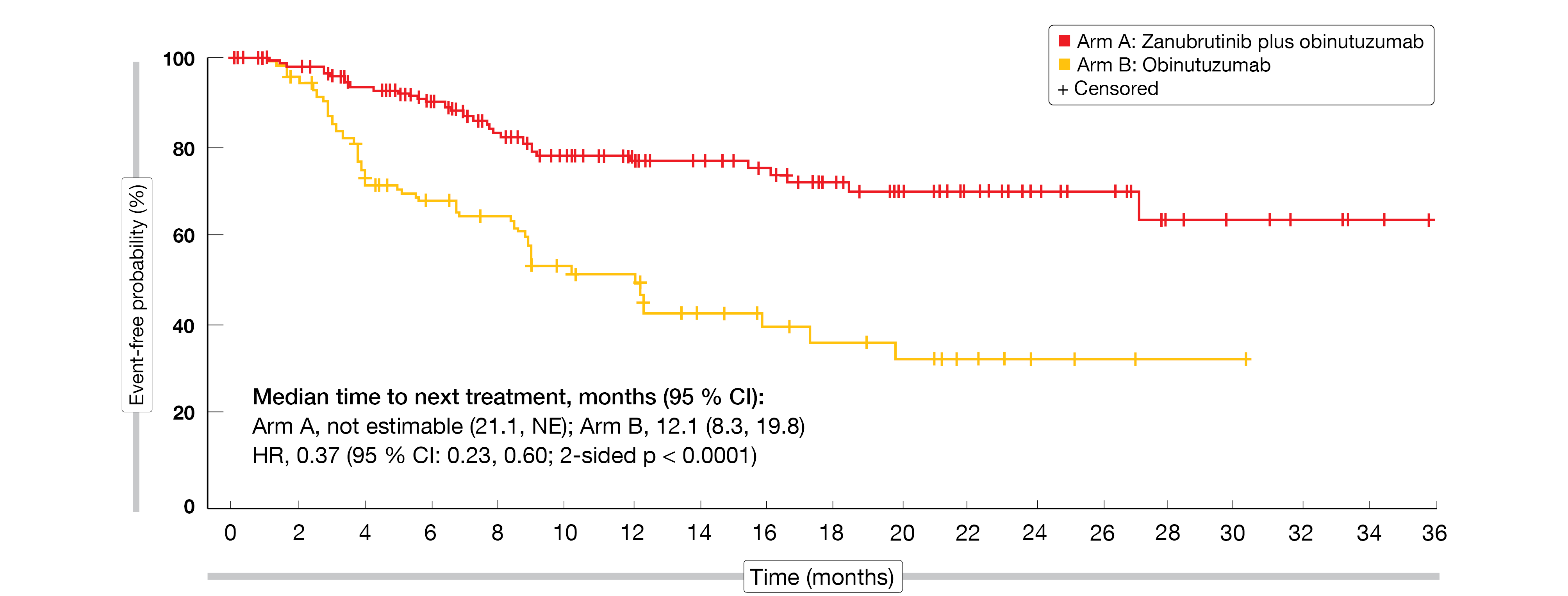
The combination conferred a 49 % reduction in the risk of progression or death compared to the monotherapy, with median progression-free survival of 27.4 vs. 11.2 months (HR, 0.51; p = 0.0040). Median duration of response had not been reached yet in either arm; at 18 months, 70.9 % vs. 54.6 % of patients responded. Time to next anti-lymphoma treatment was significantly prolonged with zanubrutinib/obinutuzumab (not reached vs. 12.1 months; HR, 0.37; p < 0.0001; Figure 1). Although ROSEWOOD was not powered to detect an overall survival difference between the regimens, the findings favored the combination over the single-agent treatment, with a 56 % reduction in mortality risk (HR, 0.44).
The safety profile of zanubrutinib/obinutuzumab was generally comparable to that of obinutuzumab monotherapy. Decreases in thrombocyte and neutrophil counts occurred most commonly, and among treatment-emergent adverse events of special interest (AESIs), infections prevailed. In their summary, the authors concluded that zanubrutinib plus obinutuzumab has a favorable benefit-risk profile and represents a potential combination therapy for patients with relapsed/refractory FL.
Figure 1: Superior time to next anti-lymphoma treatment with zanubrutinib/obinutuzumab vs. obinutuzumab monotherapy in the ROSEWOOD trial
Intermittent dosing of zandelisib
Specific pharmacologic properties such as extensive tissue distribution and prolonged tumor residence compared to plasma permit the administration of the potent and selective oral PI3Kδ inhibitor zandelisib on an intermittent dosing schedule [5]. This schedule was introduced to allow regulatory T cell repopulation and to mitigate immune-mediated AESIs without loss of disease control. A phase Ib study assessing intermittent dosing on days 1-7 of 28-day cycles revealed high ORRs with zandelisib monotherapy (77.8 %) and the combinations of zandelisib with rituximab (94.7 %) or zanubrutinib (82.1 %) in the setting of relapsed/refractory FL [6]. The regimens were generally well tolerated, with low rates of AESIs and discontinuations due to treatment-related AEs.
These findings support the ongoing open-label, global, phase II TIDAL study that is investigating single-agent zandelisib 1 week on/3 weeks off from cycle 3 after daily dosing for 2 cycles. The study population consists of patients with grade I-IIIA FL who have developed progression after ≥ 2 treatment lines including an anti-CD20 antibody and an alkylating agent. Initially, TIDAL had been a randomized trial containing a continuous dosing arm, which was closed after maturing data from the phase Ib study demonstrated improved results of the intermittent treatment. At EHA 2022, the primary analysis of TIDAL was reported [7]. The safety population comprised 121 patients, while the primary efficacy population was made up by the first 91 patients treated with intermittent dosing. ORR in the primary efficacy population constituted the primary outcome.
Substantial responses with low rates of grade 3 AESIs
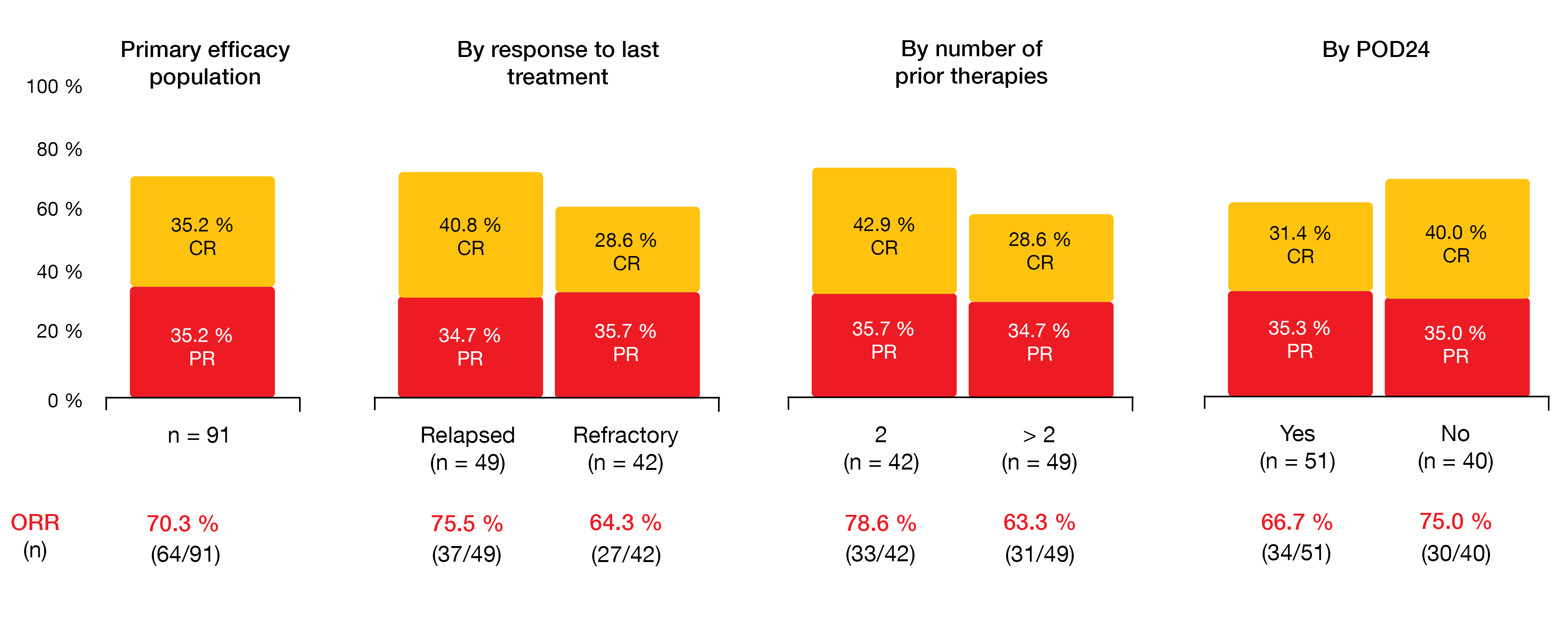
In this heavily pretreated patient group, zandelisib gave rise to an ORR of 70.3 %, including 35.2 % of complete remissions. Responses occurred early on; 87.5 % of all remissions were noted at the end of cycle 2, and 75.0 % of complete remissions had emerged until the end of cycle 4. Disease control was achieved in 85 %. ORRs did not differ according to response to the last treatment, number of prior therapies, or presence of progression of disease within 2 years (Figure 2).
Intermittent treatment with zandelisib displayed a favorable toxicity profile. The most common AEs included diarrhea (all grades, 33 %), neutropenia (26 %), and nausea (19 %). Approximately half of patients with neutropenia experienced grade 4 events, although they responded to growth factor support. Discontinuations due to treatment-related AEs were observed in less than 10 %, and the analysis demonstrated low rates of grade 3 AESIs such as diarrhea (4.9 %), rash (3.3 %), stomatitis (2.5 %), and colitis (1.7 %). The cumulative incidence of grade 3 AESIs did not exceed 20 %, with the vast majority occurring during the first 3 cycles.
As the authors emphasized, these data support the evaluation of zandelisib on intermittent dosing as a single agent or in combination in various B-cell malignancies, both in relapsed disease and in earlier lines of therapy. At present, the phase III COASTAL trial is assessing zandelisib plus rituximab vs. chemoimmunotherapy in patients with relapsed/refractory FL and marginal zone lymphoma (NCT04745832).
Figure 2: TIDAL study: overall response rates obtained with intermittent dosing of zandelisib in the overall population and in several subgroups
Parsaclisib plus obinutuzumab/bendamustine: CITADEL-102
Monotherapy with the oral, potent, highly selective next-generation PI3Kδ inhibitor parsaclisib has demonstrated rapid and durable responses in the phase II CITADEL-203 trial in the setting of relapsed/refractory FL [8]. The open-label, phase I, dose-finding CITADEL-102 study assessed parsaclisib in addition to the proven combination of obinutuzumab and bendamustine in patients with relapsed/refractory FL after pretreatment with rituximab-containing regimens. Overall, 26 patients at 15 sites in Europe and the US participated in the safety run-in and dose expansion periods.
The data suggested that parsaclisib plus obinutuzumab/bendamustine has a manageable safety profile [9]. No dose de-escalation was required, and the maximum tolerated dose of parsaclisib was not exceeded. Among any-grade AEs, pyrexia (53.8 %), neutropenia (50.0 %), and diarrhea (46.2 %) occurred most frequently. The most common grade 3/4 AE was neutropenia (34.6 %), followed by febrile neutropenia (23.1 %). Treatment-emergent AEs leading to parsaclisib treatment discontinuation and dose reductions were noted in 30.8 % and 23.1 %, respectively. Most AEs proved manageable with dose delays or reductions.
Almost all patients experienced substantial reductions from baseline in target lesions size. The ORR observed in CITADEL-102 was 76.9 %, with complete responses/complete metabolic responses in 65.4 %. Median progression-free survival, median overall survival, and median duration of response had not been reached yet. In their assessment of the study findings, the authors concluded that parsaclisib plus obinutuzumab/bendamustine showed promising efficacy in rituximab-pretreated FL patients and warrants further investigation.
REFERENCES
- Carbone A et al., Follicular lymphoma. Nat Rev Dis Primers 2019; 5(1): 83
- Rivas-Delgado A et al., Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol 2019; 184(5): 753-759
- Tam CS et al., Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv 2020; 4(19): 4802-4811
- Zinzani PL et al., Zanubrutinib plus obinutuzumab versus obinutuzumab monotherapy in patients with relapsed or refractory follicular lymphoma: primary analysis of the phase 2 randomized ROSEWOOD trial. EHA 2022, S205
- Moreno O et al., Safety, pharmacokinetics, and pharmacodynamics of ME-401, an oral, potent, and selective inhibitor of phosphatidylinositol 3-kinase P110δ, following single ascending dose administration to healthy volunteers. Clin Ther 2018; 40(11): 1855-1867
- Soumerai JD et al., Zandelisib on intermittent dosing as single agent or in combination with rituximab or zanubrutinib in relapsed/refractory follicular lymphoma: results from a multi-arm phase 1B study. EHA 2022, P1114
- Zelenetz AD et al., Efficacy and safety of zandelisib administered by intermittent dosing in patients with relapsed or refractory follicular lymphoma: primary analysis of the global phase 2 study TIDAL. EHA 2022, S208
- Lynch RC et al., Efficacy and safety of parsaclisib in patients with relapsed or refractory follicular lymphoma: primary analysis from a phase 2 study (CITADEL-203). Blood 2021; 138(Suppl 1): 813
- Hamadani M et al., A phase 1 study evaluating safety and efficacy of parsaclisib in combination with obinutuzumab + bendamustine in patients with relapsed or refractory follicular lymphoma (CITADEL-102). EHA 2022, P1104
© 2022 Springer-Verlag GmbH, Impressum