Tackling acute myeloid leukemia via diverse pathways
Mutations of FLT3 are found in approximately 30 % of patients with newly diagnosed acute myeloid leukemia (AML), with the internal tandem duplication (ITD) representing the most common type [1-4]. FLT3-ITDhigh is associated with high leukemia burden and poor prognosis [2, 5, 6].
Quizartinib has been developed as a potent and selective, second-generation type II FLT3 inhibitor [7]. The pivotal QuANTUM-First study evaluating quizartinib in the phase III setting is the first randomized trial to assess the efficacy and safety of a specific FLT3 inhibitor in patients with FLT3-ITD–positive AML up to the age of 75 years and up to 3 years of continuation therapy.
QuANTUM-First: OS difference of almost 17 months
Newly diagnosed patients aged 18-75 years with ≥ 3 % FLT3-ITD allelic frequency participated in the international QuANTUM-First trial. They started 7+3 chemotherapy during FLT3-ITD screening and were randomized on day 7. Patients in the experimental arm (n = 268) received quizartinib on days 8-21 in addition to standard induction chemotherapy (cytarabine plus daunorubicin or idarubicin for up to 2 cycles) and, after achievement of complete response (CR) or CR with incomplete hematologic recovery (CRi), quizartinib plus consolidation (i.e., high-dose cytarabine for up to 4 cycles and/or allogeneic hematopoietic stem cell transplantation). Subsequently, those in remission at the end of consolidation including allo-HSCT could continue with up to 36 cycles of quizartinib QD. Patients in the control arm (n = 271) were treated with placebo plus identical induction and consolidation strategies followed by placebo QD for up to 36 months. Overall survival (OS) constituted the primary endpoint. The disease characteristics in the two arms were as expected for a population with FLT3-ITD–positive AML and a high burden of aggressive disease.
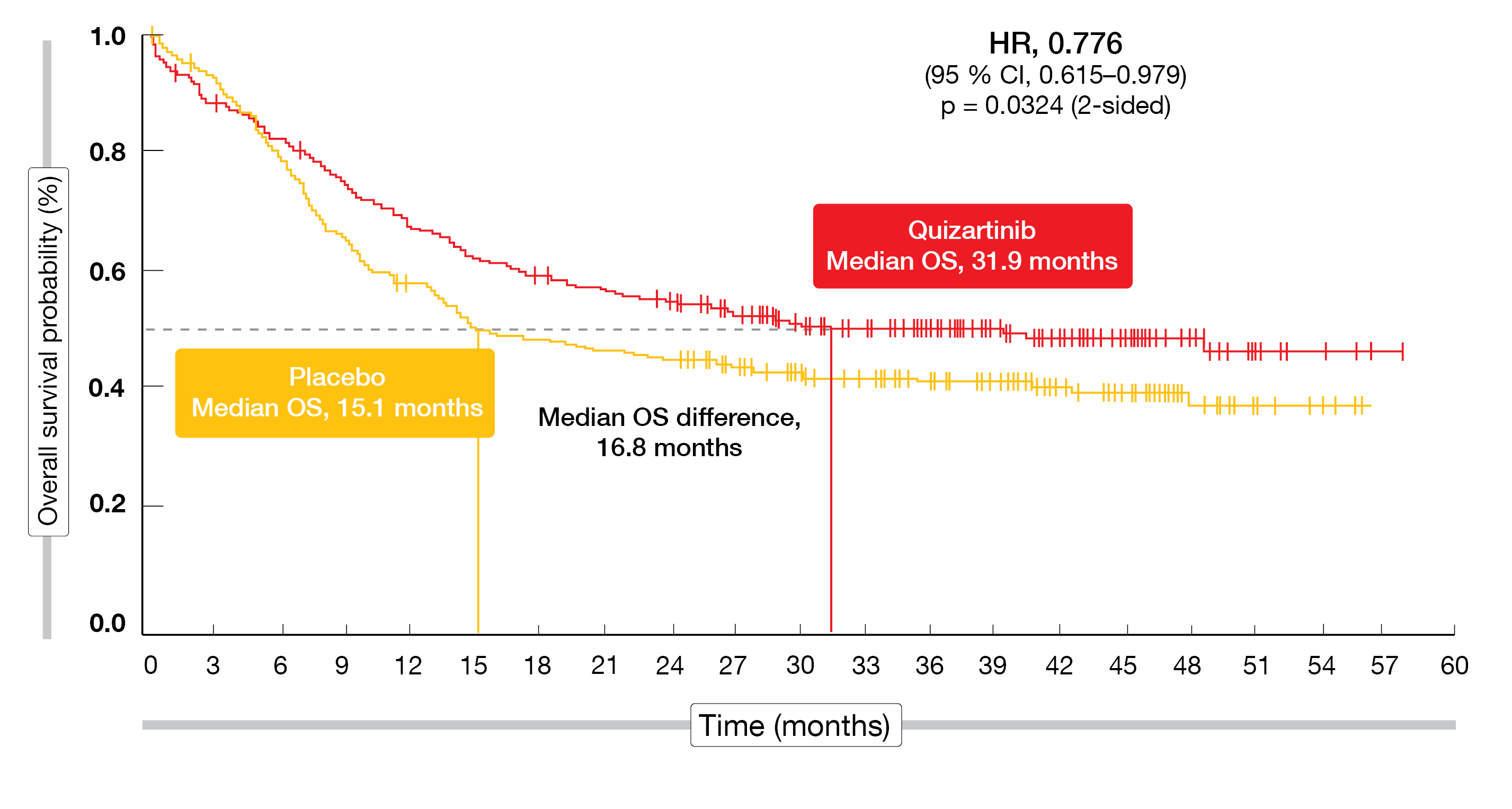
According to the analysis presented by Erba et al. at EHA 2022, the trial met its primary endpoint [8]. Median OS was more than doubled with the quizartinib-based treatment compared to standard induction and consolidation therapy alone (31.9 vs. 15.1 months; HR, 0.776; p = 0.0324; Figure 1). In the experimental and control arms, 144 and 128 patients, respectively, received allo-HSCT during the study. A sensitivity analysis in which all treated patients were censored at the time of transplant yielded an HR of 0.752 for OS, which was consistent with the primary analysis. By means of a descriptive, post-hoc analysis, OS was examined in the subgroups of patients who achieved CR during the induction phase and continued treatment with or without allo-HSCT in first remission. Here, both groups derived 40 % risk reductions when treated with quizartinib (HRs vs. placebo, 0.591 and 0.607, respectively).
Figure 1: Superior overall survival with the addition of quizartinib to standard induction and consolidation therapy in the QuANTUM-First trial
Improvements across a range of endpoints
The OS benefit was underpinned by clinically meaningful improvements in duration of CR, which was 3 times longer in the experimental arm (38.6 vs. 12.4 months), and findings in patients who achieved CR. In this group, median relapse-free survival was 39.3 vs. 13.6 months (HR, 0.613), and the cumulative incidence of relapse was lower, with 24-month rates of 31.2 % vs. 43.3 %. The CR rates themselves did not differ across the arms (54.9 % vs. 55.4 %). Also, the primary analysis for event-free survival (EFS), which required CR by day 42 of the last induction cycle, did not yield a statistically significant difference. However, prespecified sensitivity analyses of EFS (and the original definition of EFS per protocol) showed superiority of the quizartinib-based therapy regarding achievement of CR by the end of induction (HR, 0.818) as well as CR with incomplete neutrophil or platelet count recovery by the end of induction (HR, 0.729).
The safety of quizartinib combined with intensive chemotherapy and as monotherapy was generally manageable. In the experimental arm, treatment-emergent adverse events (TEAEs) led to discontinuation in 20.4 % (vs. 8.6 %) and dose reduction in 18.9 % (vs. 6.3 %). TEAEs with fatal outcome occurred in 11.3 % vs. 9.7 %. These events were mainly due to infection that emerged predominantly during the induction and consolidation phases. Infections and cytopenias represented the most common serious AEs and TEAEs associated with study drug discontinuation. Although neutropenia was observed more frequently with the quizartinib-based therapy (20.4 % vs. 10.1 %), rates for febrile neutropenia were comparable (all grades, 44.2 % vs. 42.2 %; grade ≥ 3, 43.4 % vs. 41.0 %). Non-hematologic AEs with the combined treatment included mainly pyrexia, diarrhea, hypokalemia, and nausea. QT prolongation occurred more commonly in the quizartinib arm, although most cases were mild to moderate, and proved manageable. The authors pointed out that the data obtained in the QuANTUM-First trial have the potential to change the standard of care for the treatment of adult patients with newly diagnosed FLT3-ITD–positive AML.
Unfit patients with TP53-mutated AML: magrolimab
The first-in-class anti-CD47 antibody magrolimab is a macrophage immune checkpoint inhibitor that works by blocking the inhibitory (“don’t eat me”) signal between CD47 and SIRPα, which is overexpressed in multiple cancers, including AML, and leads to macrophage immune evasion [9, 10]. Thus, magrolimab prompts macrophage activation, which enables tumor cell elimination. The rationale for the combination of this agent with the hypomethylating drug azacitidine results from the fact that azacitidine induces prophagocytic “eat me” signals, such as calreticulin, on cancer cells [11]. These signals were shown to synergize with the CD47 blockade in AML xenograft models, giving rise to enhanced phagocytosis.
Daver et al. reported phase IB results for 72 previously untreated AML patients with TP53-positive disease who received magrolimab plus azacitidine in the 5F9005 study [12]. Magrolimab was administered as a 1 mg/kg priming dose i. v. on days 1 and 4, followed by ramp-up to 30 mg/kg maintenance once or twice weekly. Azacitidine therapy entailed subcutaneous or intravenous doses of 75 mg/m2 on days 1-7 of each cycle. The study population was ineligible for intensive induction chemotherapy with cytarabine and anthracycline. Almost 80 % were ≥ 65 years old. Adverse cytogenetic risk factors and underlying myelodysplasia were present in 79.2 % and 47.2 %, respectively. Median TP53 variant allele frequency (VAF) was 38.
Encouraging remissions and OS
Magrolimab plus azacitidine demonstrated an acceptable safety profile and promising efficacy consistent with the synergy observed in the preclinical setting. The most commonly reported AEs included constipation, diarrhea, febrile neutropenia, and nausea. Grade 3 anemia was seen in 26.4 %, with grade 4 events in 2.8 % regardless of attribution. All-grade infusion-related reactions occurred in 22.2 %, while grade ≥ 3 reactions were rare at 1.4 %. Notably, no patient required dose reductions, and dose delays were reported in 45.8 %. TEAEs led to discontinuation of magrolimab and azacitidine in 30.6 % and 29.2 %, respectively. Eighteen percent of patients died within 60 days of the first study drug dose. However, the authors pointed out that early mortality is inherently high in the setting of TP53-mutated AML, with studies into azacitidine/decitabine and venetoclax reporting 20-25 % early mortality in this specific molecular subset of patients, a large proportion of this being due to refractory disease and more profound cytopenias.
Magrolimab plus azacitidine induced encouraging response rates. Almost 50 % of patients responded and 33.3 % developed CR, with half of these achieving MRD negativity. CR/CRi resulted in 41.7 %. Median duration of response was encouraging for TP53-mutated patients at 8.7 months, and median progression-free survival amounted to 7.3 months. Whole exome sequencing plus best overall response data were available for 13 patients. In this group, TP53 VAF decreased in most individuals; 7 of 9 longitudinally evaluable responders obtained TP53 VAF < 0.07 by the end of cycle 4 of combination therapy. Moreover, the analysis showed promising OS for TP53-mutated AML, with a median of 10.8 months. Among patients who were transfusion-dependent at baseline, 29.7 % and 45.8 % converted to red blood cell and platelet transfusion independence, respectively.
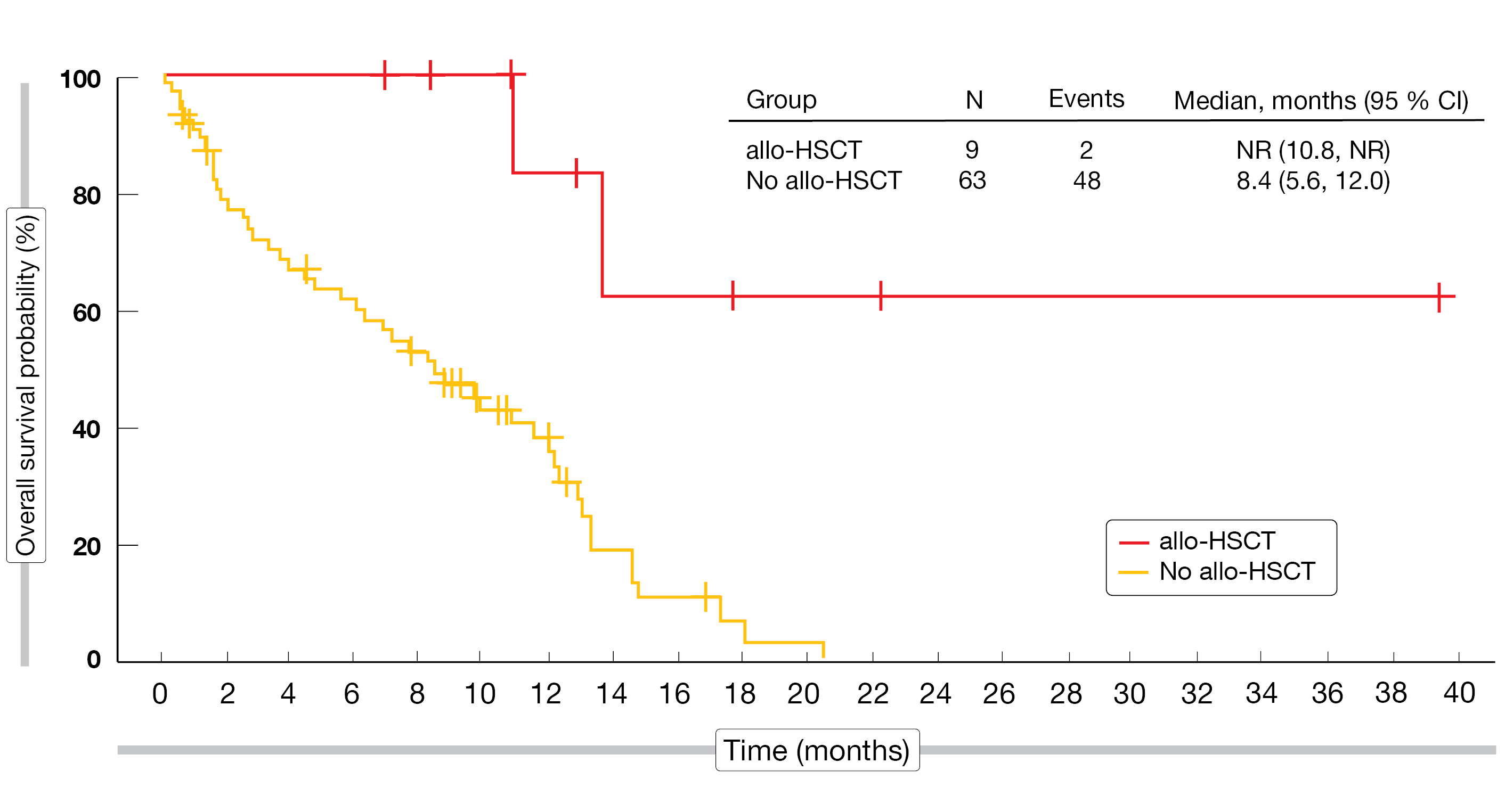
Patients who were able to progress to allo-HSCT experienced particularly favorable outcomes. Median OS after allo-HSCT had not been reached yet (vs. 8.4 months without allo-HSCT), and 83 % of the transplanted individuals were alive at 1 year (vs. 36 %; Figure 2). According to the authors, performing transplantation as soon as possible after achieving at least remission in the bone marrow appears to be the preferable treatment path on the road to long-term survival. Magrolimab plus azacitidine as frontline strategy is currently being assessed in patients with TP53-mutated AML in the phase III ENHANCE-2 trial (NCT04778397).
Figure 2: Survival advantage after magrolimab followed by allogeneic stem cell transplant (allo-HSCT) vs. patients who were ineligible for allo-HSCT
STIMULUS-AML1: sabatolimab plus venetoclax/azacitidine
Another novel target is TIM-3, an immune-myeloid regulator that is expressed on both immune cells and leukemic stem cells in myeloid malignancies [13]. The anti-TIM-3 antibody sabatolimab has a potential dual mechanism to combat AML and myelodysplastic syndrome (MDS) by boosting the ability of the immune system to eliminate leukemic stem cells and blasts, and by directly targeting TIM-3–positive leukemic stem cells [14, 15]. The combination of sabatolimab with hypomethylating agents has shown durable responses in patients with higher-risk AML and MDS in a phase IB study [14].
Sabatolimab plus azacitidine and venetoclax is being investigated in the single-arm, phase II STIMULUS-AML1 trial in patients with newly diagnosed AML who are not fit for intensive chemotherapy. Cohorts 1 and 2 received sabatolimab 400 mg and 800 mg Q4W, respectively, in addition to azacitidine and venetoclax. After the 800 mg dose had proven safe, the trial progressed to the expansion part testing the combination treatment based on the higher sabatolimab dose. At EHA 2022, preliminary findings from the completed dose-escalation part of the study (n = 18) were presented [16].
According to this, the overall safety and tolerability of the regimen were comparable to the previously reported safety profile of venetoclax and azacitidine, and there were no notable differences across the 400 mg and 800 mg dose levels. Among AEs suspected to be treatment-related, grade 3/4 neutropenia was observed most frequently, followed by grade 3/4 thrombocytopenia, grade 1/2 constipation, and anemia that was mainly grade 3/4. Three patients discontinued sabatolimab therapy due to treatment-related events. AEs leading to venetoclax and azacitidine treatment discontinuation emerged in two cases each. No dose reduction of sabatolimab was required, and only one dose-limiting toxicity (i.e., troponin T elevation suggesting asymptomatic myocarditis) occurred.
The sabatolimab-based therapy conferred preliminary clinical responses. In Cohort 1, 1 patient developed CR, while 3 had CRi, and 1 experienced disease stabilization. Among the 13 patients included in Cohort 2, 3 experienced CR, 5 CRi, and 1 morphological leukemia-free state (MLFS). Stable disease was reported in 2 patients. At present, accrual in the expansion cohort is ongoing.
BGB-11417 combined with azacitidine
Venetoclax-based therapies have improved outcomes in patients with AML who are ineligible for induction chemotherapy, although concerns regarding disease resistance and toxicities remain [17]. BGB-11417 is a potent and highly selective investigational BCL2 inhibitor that has shown superior antitumor activity compared with venetoclax in the preclinical setting and a tolerable safety profile at doses of up to 640 mg in a phase I monotherapy study [18, 19]. The ongoing, phase IB/II dose-finding and dose-expansion study BGB-11417-103 is evaluating the safety and efficacy of BGB-11417 plus azacitidine in the setting of AML. In the dose-escalation part, 3 dose levels of BGB-11417 (40 mg, 80 mg, 160 mg) were tested for 10 days after a 4-day ramp-up in cycle 1; in addition, the 160 mg dose is being assessed for 28 days.
Shortt et al. reported preliminary data obtained with the 10-day doses in 19 treatment-naïve AML patients unfit for intensive chemotherapy and in 12 patients with relapsed/refractory AML who had not received prior BCL2 inhibitor or azacitidine treatment [20]. Across the 3 dose levels, BGB-11417 was well tolerated and active in combination with azacitidine. Dose-limiting toxicities occurred in 2 patients, and 4 patients discontinued study treatment due to AEs. At 55 %, neutropenia represented the most common grade ≥ 3 AE, followed by thrombocytopenia (48 %) and anemia (42 %). Neutropenia proved manageable with growth factor support and dose modifications.
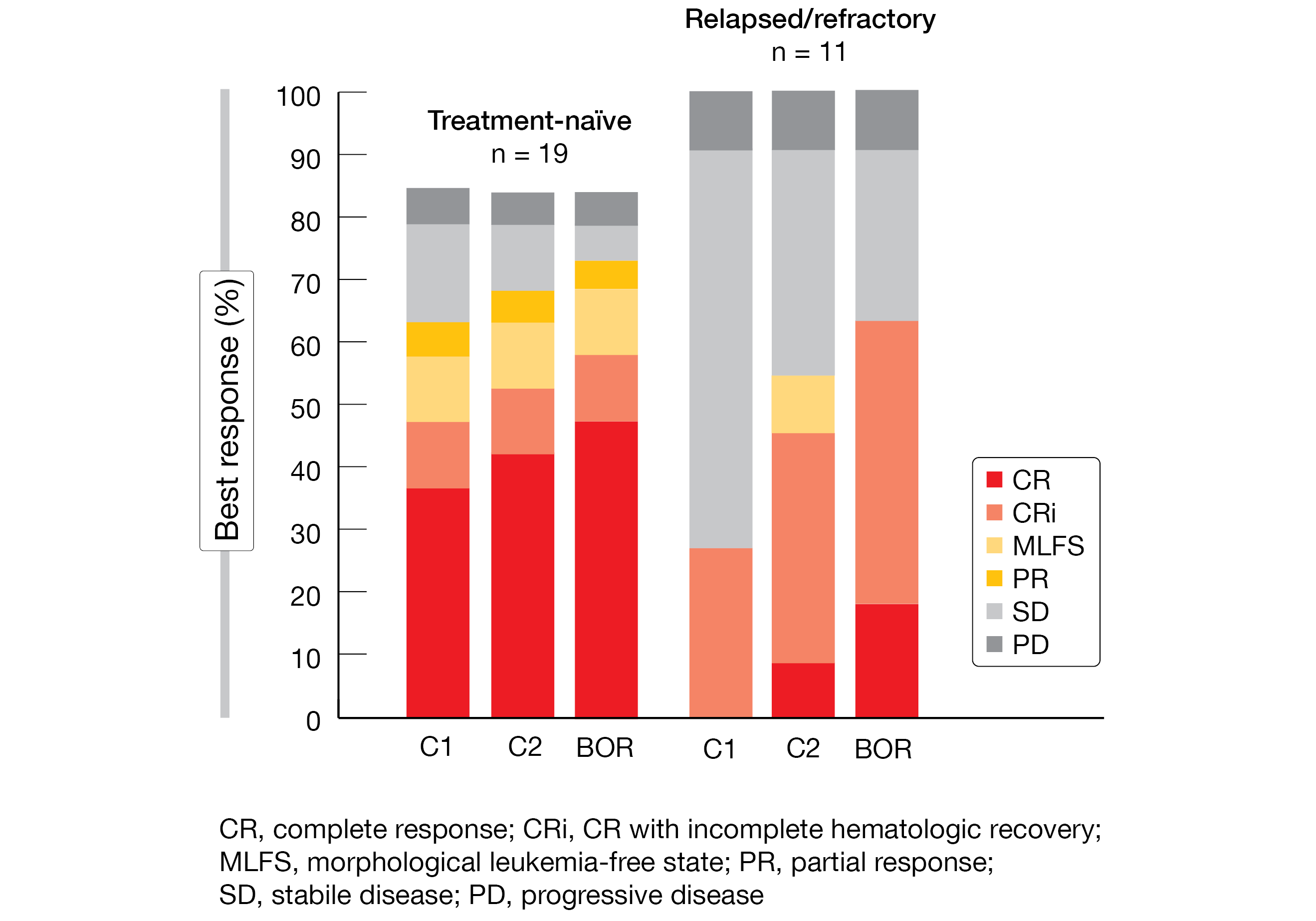
Overall responses, which were a composite of CR, CRi, MLFS and partial response, resulted in 74 % and 64 % of treatment-naïve and relapsed/refractory patients, respectively (Figure 3). Within these groups, 58 % and 55 % of patients, respectively, met the criteria for CR plus CR with partial hematological recovery. Most CRs were achieved by the end of cycle 1. Nearly 40 % of 13 patients with CR/CRi who had evaluable flow cytometry results obtained MRD negativity. Enrollment in the safety expansion is ongoing, and evaluation of the 28-day dosing regimen is planned.
Figure 3: Best overall response (BOR) and responses by cycle 1 and 2 with BGB-11417 plus azacitidine in patients with treatment-naïve and relapsed/refractory AML
REFERENCES
- O’Donnell MR et al., Acute myeloid leukemia, version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15(7): 926-57
- Levis M, FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematol Am Soc Hematol Educ Program 2013; 2013: 220-226
- Kottaridis PD et al., Flt3 mutations and leukaemia. Br J Haematol 2003; 122(4): 523-538
- Nagel G et al., Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol 2017; 96(12): 1993-2003
- Ding L et al., Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012; 481(7382): 506-510
- Grimwade D, Mrozek K. Diagnostic and prognostic value of cytogenetics in acute myeloid leukemia. Hematol Oncol Clin North Am 2011; 25(6): 1135-1161, vii
- Zarrinkar P et al., AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia. Blood 2009; 114(14): 2984-2992
- Erba HP et al., Quizartinib prolonged survival vs placebo plus intensive induction and consolidation therapy followed by single-agent continuation in patients ages 18-75 years with newly diagnosed FLT3-ITD+ AML. EHA 2022, S100
- Liu J et al., Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One 2015; 10(9): e0137345
- Chao MP et al., Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol 2020; 9: 1380
- Feng D et al., Combination treatment with 5F9 and azacitidine enhances phagocytic elimination of acute myeloid leukemia. Blood 2018; 132(1 Suppl): 2729
- Daver NG et al., Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in frontline patients with TP53-mutated acute myeloid leukemia: phase 1b results. EHA 2022, S132
- Das M et al., Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev 2017; 276(1): 97-111
- Brunner AM et al., Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents in patients with very high/high-risk myelodysplastic syndrome and acute myeloid leukemia: final analysis from a phase Ib study. ASH 2021, abstract 244
- Acharya N et al., Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer 2020; 8(1): e00091
- Zeidan AM et al., First results of a phase II study (STIMULUS-AML 1) investigating sabatolimab + azacitidine + venetoclax in patients with newly diagnosed acute myeloid leukemia. EHA 2022, P582
- DiNardo CD et al., Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 2020; 383(7): 617-629
- Hu N et al., Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. Cancer Res 2020; 80(suppl 16): 3077
- Opat S et al., A phase 1 study with the novel B-cell lymphoma inhibitor BGB-11417 as monotherapy or in combination with zanubrutinib in patients with B-cell malignancies: preliminary data. EHA 2022, P687
- Shortt J et al., Preliminary safety and efficacy of BGB-1147, a potent and selective B-cell lymphoma 2 inhibitor in patients with acute myeloid leukemia, EHA 2022, P590
© 2022 Springer-Verlag GmbH, Impressum