Waldenström macroglobulinemia: findings from ASPEN and BRUIN
ASPEN: HRQoL for zanubrutinib vs. ibrutinib
In the management of patients with Waldenström macroglobulinemia (WM), BTK inhibitors have changed the therapeutic landscape and are considered preferred treatment options for the first and later lines [1]. Compared to the first-in-class agent ibrutinib, the potent and irreversible BTK inhibitor zanubrutinib offers improved BTK selectivity that minimizes off-target effects and toxicities [2]. Zanubrutinib 160 mg BID was compared with ibrutinib 420 mg OD until progression in the open-label, randomized, phase III ASPEN trial in patients with relapsed/refractory or treatment-naïve WM harboring activating MYD88 mutations. Analyses have previously demonstrated deep and durable responses with zanubrutinib compared to ibrutinib, as well as an improved safety/tolerability profile [3, 4].
The investigators assessed health-related quality of life (HRQoL) exploratory endpoints via patient-reported outcome data based on the EORTC QLQ-C30 and the EQ-5D-5L visual analog scale. At EHA 2023, Tedeschi et al. reported the results for both the intent-to-treat (ITT) population and patients achieving very good partial response (VGPR) by cycle 25 [5]. The ITT population consisted of 102 vs. 99 patients treated with zanubrutinib vs. ibrutinib, while in the group with VGPR, 31 (38.2 %) and 17 (25.3 %) had received zanubrutinib and ibrutinib, respectively. Median time to VGPR was shorter with zanubrutinib (8.3 vs. 16.6 months). No patient in either arm achieved complete remission.
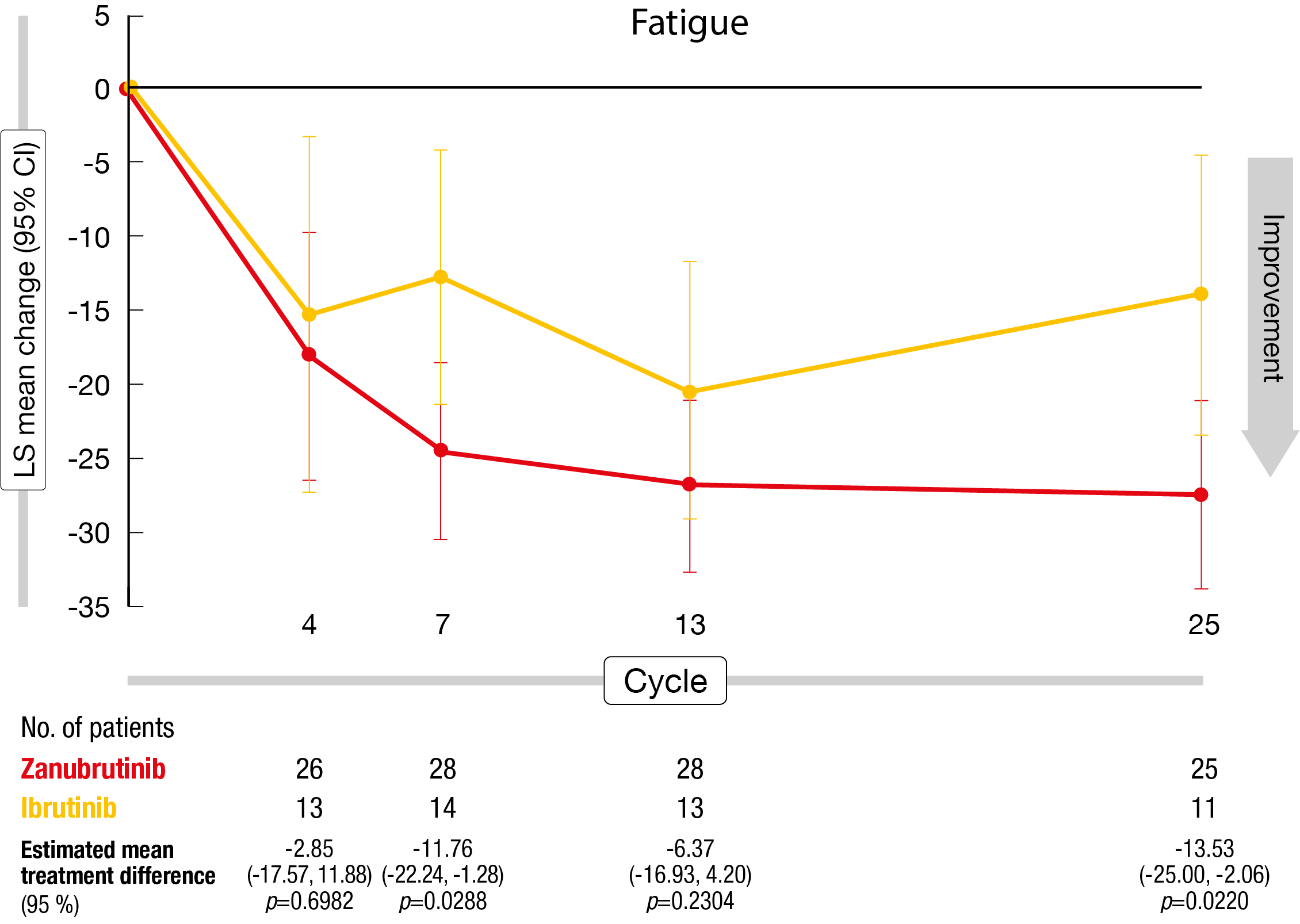
Both in the ITT population and the patients with VGPR by cycle 25, zanubrutinib therapy gave rise to clinically meaningful differences for diarrhea and nausea/vomiting at cycle 4 as the symptom scores remained stable in the experimental arm but showed initial worsening in the ibrutinib arm (Figure). Regarding other endpoints, the differences were not significant, with both arms showing improvements. In the group of patients with VGPR, the zanubrutinib-treated cohort generally experienced greater functional and symptomatic improvements than the ibrutinib group. Differences for physical functioning and fatigue were clinically meaningful at cycles 7 and 25.
According to the authors, the improved HRQoL seen at cycle 25 in the zanubrutinib-treated VGPR group is consistent with the shorter median time to VGPR and suggests that when the disease is controlled to a similar extent, patients on zanubrutinib fare better in terms of overall HRQoL than those on ibrutinib. Moreover, the EQ-5D-5L visual analog scale analysis showed that improvements from baseline were consistently greater in the zanubrutinib arm both in the ITT and VGPR populations. Overall, these findings support the use of zanubrutinib as an effective BTK inhibitor therapy option in patients with WM.
Figure: Least-squares mean change from baseline in the fatigue symptom score with zanubrutinib vs. ibrutinib (population with very good partial response by cycle 25)
Pirtobrutinib: BRUIN study
Progression and intolerance frequently necessitate discontinuation of covalent BTK inhibitor therapy with ibrutinib, leaving the patients with an unfavorable prognosis [6-9]. The highly selective, non-covalent (reversible) BTK inhibitor pirtobrutinib has been designed to inhibit both wildtype and C481-mutant BTK with equal low nM potency and has favorable oral pharmacology that enables continuous BTK inhibition throughout the dosing interval regardless of the intrinsic BTK turnover rate, thus likely defying resistance mechanisms to BTK inhibition [10, 11].
The phase I/II BRUIN study is evaluating pirtobrutinib in various B-cell malignancies including WM (n = 80); this cohort contains 63 patients pretreated with covalent BTK inhibitors (cBTKi) as well as 17 cBTKi-naïve individuals. The median number of prior lines of systemic therapy in these two groups is 3 and 2, respectively. Chemotherapy has been administered in 83 % and 100 %, respectively, and anti-CD20 antibody treatment in 92 % and 94 %, respectively. Scarfò et al. presented efficacy and safety results at EHA 2023 [12].
The major response rates for cBTKi-pretreated and cBTKi-naïve patients were 66.7 % and 88.2 %, respectively. Although no complete remissions emerged, the cBTKi-pretreated group achieved a notable VGPR rate of 23.8 %. In this cohort, median overall survival had not been reached yet, and progression-free survival was 19.4 months. At 18 months, 57.1 % of patients were alive and progression-free. Patients who were cBTKi-naïve achieved a VGPR rate of 29.4 %.
In the safety population of the BRUIN study (n = 773), treatment-related adverse events mainly included bruising (all grades, 15.1 %), neutropenia (14.7 %) and contusion (12.8 %), with few grade ≥ 3 events. The safety profiles in the overall and WM populations were generally consistent. cBTKi-associated side effects such as hemorrhage, hypertension and atrial fibrillation/flutter were infrequent. Discontinuations and dose reductions due to treatment-related adverse events occurred in 2.6 % and 4.5 % of all patients, respectively. As the authors noted, pirtobrutinib showed promising efficacy in this heavily pretreated relapsed/refractory WM patients and continued to be well-tolerated.
REFERENCES
- NCCN Clinical Practice Guidelines in Oncology, Waldenström Macroglobulinemia/Lymphoplasmacytic Lymphoma. V1.2023
- Guo Y et al., Discovery of zanubrutinib (BGB-3111),
a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem 2019; 62(17): 7923-7940 - Tam CS et al., A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 2020; 136(18): 2038-2050
- Dimopoulos M et al., ASPEN: Long-term follow-up results of a phase 3 randomized trial of zanubrutinib vs ibrutinib in patients with Waldenström macroglobulinemia. Hemasphere 2022; 6(Suppl): 1048-1049
- Tedeschi A et al., Health-related quality of life in patients with Waldenström macroglobulinemia treated with zanubrutinib vs ibrutinib: results from the phase 3 ASPEN trial long-term follow-up. EHA 2023, abstract P1679
- Coutre SE et al., Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv 2019; 3(12): 1799-1807
- Cheah CY et al., Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 2015; 26(6): 1175-1179
- Martin P et al., Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016; 127(12): 1559-1563
- Rai S et al., MCL-041: Outcomes for recurrent mantle cell lymphoma post-BTK inhibitor therapy in Japan: An administrative database study. Clin Lymphoma Myeloma Leuk 2021; 21(Suppl 1): S407-S408
- Mato A et al., Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet 2021; 397(10277): 892-901
- Brandhuber B et al., LOXO-305, a next generation reversible BTK inhibitor, for overcoming acquired resistance to irreversible BTK inhibitors. Clin Lymphoma Myeloma Leuk 2018; 18(Suppl 1): S216
- Scarfò L et al., Efficacy of pirtobrutinib, a highly selective, non-covalent (reversible) BTK inhibitor in relapsed/refractory Waldenström macroglobulinemia: results from the phase 1/2 BRUIN study. EHA 2023, abstract P1108
© 2023 Springer-Verlag GmbH, Impressum