Looking more closely at upcoming and established immunotherapy standards
IMpower010:
adjuvant atezolizumab
Adjuvant treatment using immune checkpoint inhibition after complete resection of early-stage lung cancer is being investigated considering the modest survival benefit conferred by platinum-based combination chemotherapy in this setting [1, 2]. IMpower010 was the first phase III immunotherapy study to demonstrate a significant disease-free survival (DFS) improvement in the adjuvant setting after platinum-based chemotherapy [3]. Patients included in this trial had undergone complete resection of stage IB-IIIA NSCLC and subsequently received 1–4 cycles of cisplatin-based chemotherapy. Three to 8 weeks after the last dose, 1,005 patients were randomized to either atezolizumab 1,200 mg 3-weekly for 16 cycles or best supportive care (BSC). DFS was tested hierarchically in the PD-L1 TC ≥ 1 % stage II-IIIA population followed by the all-randomized stage II-IIIA group and the ITT (i.e., stage IB-IIIA) population.
At the time of the interim analysis, atezolizumab gave rise to a significant DFS benefit in the PD-L1 TC ≥ 1 % stage II-IIIA group, leading to a 34 % risk reduction (median DFS, not estimable vs. 35.3 months; HR, 0.66; p = 0.0039) [3]. DFS rates at 36 months were 60.0 % vs. 48.2 %. The greatest magnitude of DFS improvement, however, occurred in the stage II-IIIA subpopulation with high PD-L1 expression (TC ≥ 50 %). Felip et al. presented further analyses for this cohort at the ELCC 2022 [4].
Reduction of distant relapses
Median DFS in the group with PD-L1 TC ≥ 50 % stage II-IIIA disease had not been reached yet with atezolizumab and was 35.7 months with BSC, translating into a 57 % reduction in the risk of disease recurrence or death (HR, 0.43). At 36 months, 73.8 % vs. 48.6 % of patients were disease-free. Similar results emerged after the exclusion of patients with EGFR and ALK alterations (median DFS, not estimable vs. 37.3 months; HR, 0.43). Exploratory overall survival (OS) data for these groups were immature, and further follow-up is required. Most key subgroups within the PD-L1 TC ≥ 50 % stage II-IIIA population fared better with adjuvant atezolizumab than with BSC regarding DFS. The risk reductions achieved by these subgroups were similar after the exclusion of EGFR– and ALK-positive patients.
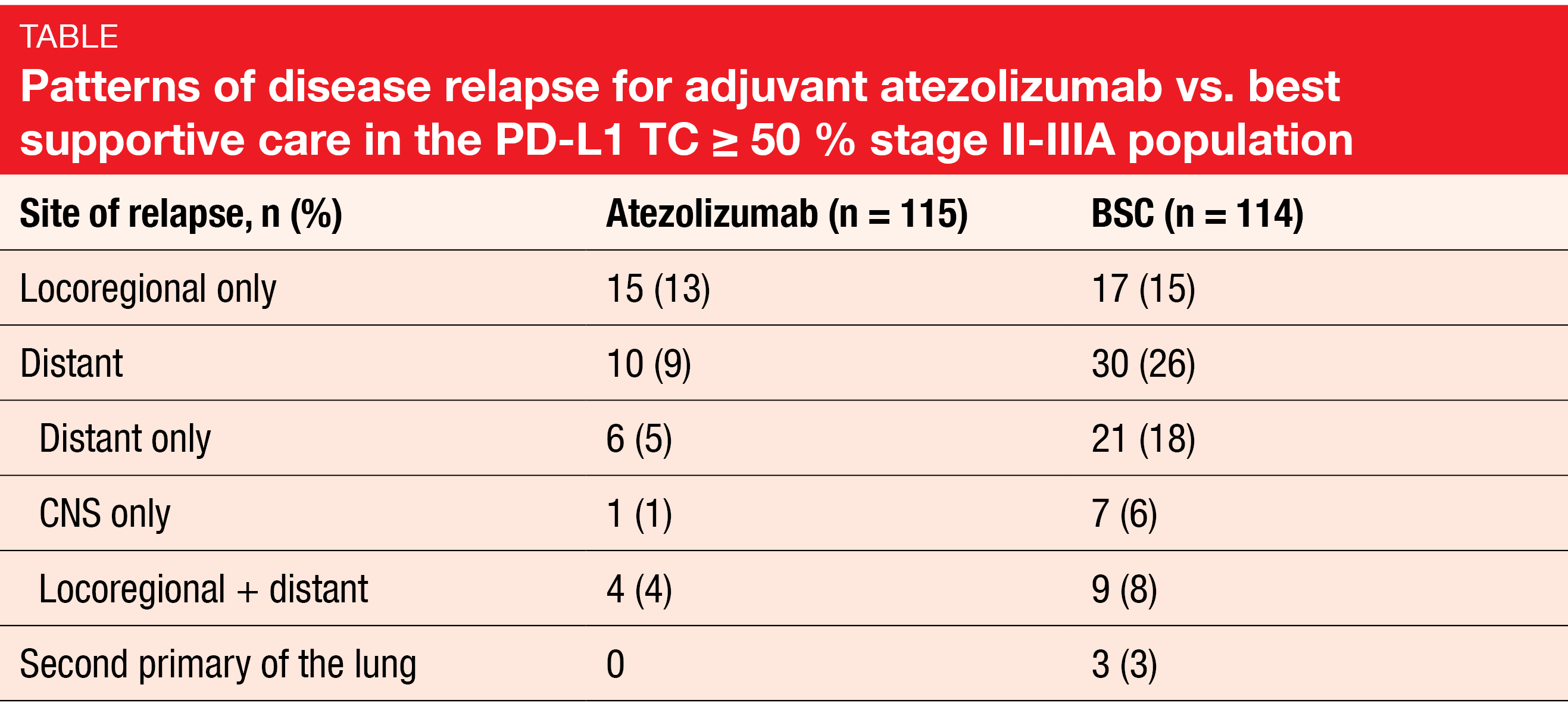
The percentage of patients experiencing relapse as their earliest DFS event was halved in the PD-L1 TC ≥ 50 % stage II-IIIA population for atezolizumab vs. BSC (22 % vs. 44 %). Moreover, the relapse patterns differed by site. While locoregional progression as a sole event occurred in comparable proportions of patients across the two arms (13 % vs. 15 %), distant relapses were markedly less frequent with atezolizumab. Five percent vs. 18 % of patients treated with atezolizumab and BSC, respectively, only experienced distant recurrence. In addition, lower rates emerged in the experimental arm with respect to CNS recurrence, locoregional plus distant relapse, and second primaries of the lung (Table). Time to relapse was 18.1 vs. 10.1 months for atezolizumab vs. BSC. Any systemic post-relapse treatment was administered in 76 % and 60 % of patients, respectively, with 16 % and 38 % receiving immunotherapy.
The safety results observed in the PD-L1 TC ≥ 50 % stage II-IIIA group were consistent with those for the stage IB-IIIA population. No patient died due to treatment-related AEs (TRAEs; vs. 0.8 % for atezolizumab in the overall population), and AEs leading to discontinuation of atezolizumab emerged in 19 % (vs. 18.2 %). According to the authors, these findings build on the positive benefit-risk profile for atezolizumab in PD-L1–expressing NSCLC and support its use as adjuvant treatment.
3-year follow-up for KEYNOTE-598
In the randomized, double-blind, phase III KEYNOTE-598 trial, the first-line regimen of pembrolizumab plus ipilimumab did not improve efficacy compared to single-agent pembrolizumab while increasing toxicity in stage IV NSCLC with PD-L1 TPS ≥ 50 % [5]. This led to discontinuation of both ipilimumab and placebo per external data monitoring committee recommendation. Pembrolizumab monotherapy continued in both arms. Each arm contained approximately 280 patients; 33.8 % and 38.7 % received subsequent anticancer treatment in the experimental and control arms, respectively.
Long-term outcomes presented by Rodríguez-Abreu et al. at the ELCC after 13 additional months of follow-up confirmed the absence of clinical benefits with the combination [6]. Both OS and PFS curves were superimposable across the treatment regimens, with HRs of 1.05 and 0.99, respectively. At 24 months, 48.0 % and 48.5 % of patients, respectively, were alive, with 27.2 % and 25.1 %, respectively, being progression-free. Likewise, ORRs did not differ (46.5 % vs. 46.1 %). Even with the prolonged follow-up, the incidence of TRAEs was higher in the combination arm (any grade, 75.5 % vs. 68.7 %; grade 3-5, 35.1 % vs. 20.3 %), leading more often to treatment discontinuation. Patients treated with pembrolizumab/ipilimumab received 10 cycles on average, while those in the pembrolizumab monotherapy arm received 15 cycles.
Furthermore, the scientists investigated clinical outcomes in patients who completed 35 cycles, i.e., approximately 2 years of pembrolizumab treatment. This applied to more patients in the monotherapy arm (n = 71) than in the combination arm (n = 52). Most of these had durable responses, including patients who discontinued ipilimumab after the first interim analysis. Compared to the overall population, both groups showed higher ORRs of approximately 88 %. Median duration of response had not been reached yet in either arm. Two patients in the combination arm and 9 in the monotherapy arm started a second course of pembrolizumab. At data cutoff, 8 of them were still alive. Overall, these findings underscore the significance of single-agent pembrolizumab as a standard-of-care therapy for patients with metastatic NSCLC and PD-L1 TPS ≥ 50 % who do not harbor targetable EGFR or ALK aberrations.
Camrelizumab in squamous tumors: CameL-sq
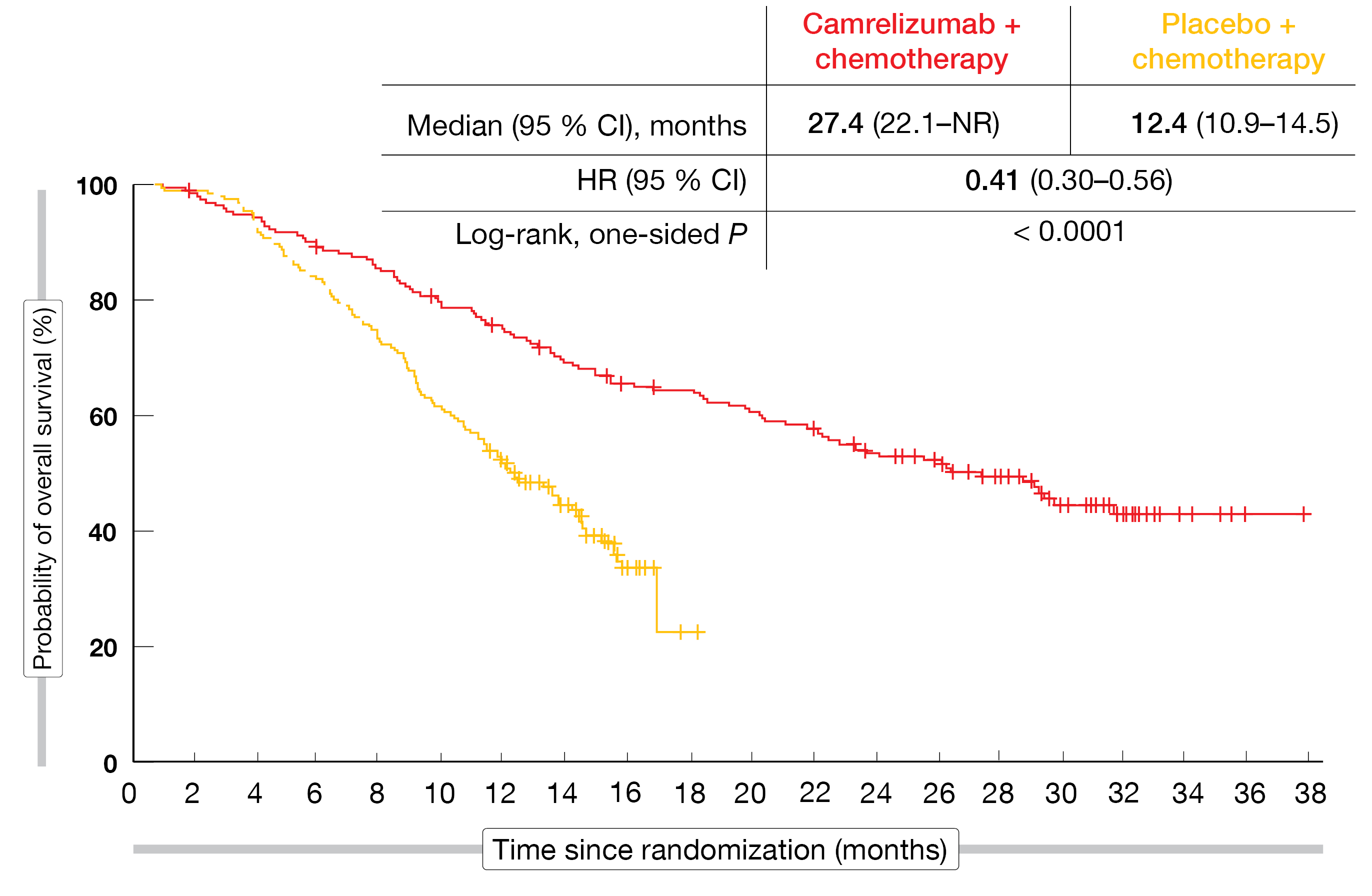
The anti-PD-1 checkpoint inhibitor camrelizumab was evaluated as first-line treatment in the randomized, phase III CameL-sq trial conducted in Chinese patients with stage IIIB-IV squamous NSCLC. Patients were randomized to either camrelizumab 200 mg plus carboplatin/paclitaxel (n = 193) or placebo plus carboplatin/paclitaxel (n = 196) 3-weekly for 4–6 cycles. This was followed by maintenance treatment with camrelizumab 200 mg 3-weekly in the experimental arm, while the control arm received placebo. Cross-over to the active treatment was permitted upon progression. At the time of the primary analysis, the camrelizumab-based regimen, as compared to chemotherapy alone, gave rise to a significant PFS improvement (8.5 vs. 4.9 months; HR, 0.37; p < 0.0001) [7]. OS results were immature. According to the update reported at the ELCC by Zhou et al., the addition of camrelizumab to chemotherapy continued to demonstrate survival benefits after > 1 year of additional follow-up [8]. Despite a cross-over rate of 55.8 %, median OS was almost double in the experimental arm, translating into a 43 % reduction in mortality risk (27.4 vs. 15.5 months; HR, 0.57; p < 0.0001). At 36 months, 42.8 % vs. 23.7 % of patients were alive. A rank-preserving structural failure time model was used to estimate the cross-over–adjusted OS, resulting in a 59 % mortality reduction with camrelizumab plus chemotherapy (27.4 vs. 12.4 months; HR, 0.41; p < 0.0001; Figure). No new safety signals emerged over time. The authors concluded that these data further support camrelizumab plus carboplatin/paclitaxel as a standard first-line option for patients with advanced squamous NSCLC.
Figure: CameL-sq trial: overall survival with camrelizumab plus chemotherapy vs. chemotherapy alone after adjustment for cross-over
Tislelizumab: safety data from RATIONALE-307
Tislelizumab is a new PD-1 inhibitor that was designed to minimize binding to Fcγ receptors on macrophages to abrogate antibody-dependent phagocytosis, which is a potential mechanism of resistance to anti-PD-1 therapy [9, 10]. The open-label, randomized, phase III RATIONALE-307 trial compared tislelizumab plus paclitaxel/carboplatin (Arm A) with tislelizumab plus nab-paclitaxel/carboplatin (Arm B) and paclitaxel/carboplatin alone (Arm C) as first-line treatment for patients with locally advanced or metastatic squamous NSCLC. Tislelizumab plus chemotherapy significantly prolonged PFS (7.6 months in both Arm A and B) vs. chemotherapy (Arm C: 5.5 months; p < 0.001 for both comparisons), which translated into risk reductions of 48 % and 52 %, respectively (HRs, 0.524 and 0.478, respectively) [11]. Also, a manageable safety and tolerability profile was observed. At ELCC 2022, Yu et al. presented results from a post-hoc safety analysis of the RATIONALE-307 study that included a total of 355 patients [12].
These data showed that in patients with advanced squamous NSCLC, tislelizumab plus chemotherapy had a tolerable safety profile which was consistent with that of other checkpoint inhibitors including PD-1 inhibitors. Endocrine disorders were more common in Arms A and B (12.5 % and 6.8 %) than in Arm C (0 %), as were hypersensitivity reactions (25.8 % and 30.5 % vs. 12.0 %) and hypothyroidism (13.3 % and 14.4 % vs. 2.6 %). Regarding the most commonly reported treatment-emergent AEs by system organ class, comparable rates resulted for tislelizumab plus chemotherapy vs. chemotherapy alone, indicating that tislelizumab did not compound chemotherapy-specific toxicity.
REFERENCES
- Arriagada R et al., Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004; 350(4): 351-360
- Pignon JP et al., Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008; 26(21): 3552-3559
- Felip E et al., Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial Lancet 2021; 398(10308): 1344-1357
- Felip E et al., Atezolizumab vs best supportive care in stage II-IIIA NSCLC with high PD-L1 expression: sub-analysis from the pivotal phase III IMpower010 study. ELCC 2022, abstract 80O
- Boyer M et al., Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 Tumor Proportion Score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol 2021; 39(21): 2327-2338
- Rodríguez-Abreu D et al., Pembrolizumab plus ipilimumab or placebo in previously untreated metastatic NSCLC with PD-L1 tumor proportion score ≥ 50 %: KEYNOTE-598 3-year follow-up. ELCC 2022, abstract 6M0
- Ren S et al., Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J Thorac Oncol 2022; 17(4): 544-557
- Zhou C et al., First-line camrelizumab plus carboplatin and paclitaxel for advanced squamous non-small-cell lung cancer. ELCC 2022, abstract 3M0
- Dahan R et al., FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 2015; 28(3): 285-295
- Zhang T et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090
- Wang J et al., Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol 2021; 7(5): 709-717
- Yu X et al., RATIONALE-307: safety analysis of patients receiving tislelizumab plus chemotherapy versus chemotherapy alone in advanced squamous NSCLC. ELCC 2022, abstract 18P
© 2022 Springer-Verlag GmbH, Impressum