Towards treating all stages of gastric cancer and gastroesophageal junction adenocarcinoma
Gastric and esophagus cancers were the fifth and the seventh most frequently diagnosed cancers worldwide in 2020, respectively. In the same year, these cancers ranked fourth and sixth in mortality, respectively [1]. Most patients with gastric cancer/gastroesophageal junction adenocarcinoma (GC/GEJA) present with inoperable or metastatic disease at the time of diagnosis; resulting in a strong need for efficient and tolerable first-line (1L) and second-line (2L) treatments [2].
Moonlight study: 1L FOLFOX induction vs combination therapy
The combination of leucovorin, fluorouracil (5-FU) and oxaliplatin (FOLFOX) plus nivolumab, an anti-PD-1 immune checkpoint inhibitor (ICI), has become the standard-of-care for first-line therapy of patients with esophagogastric adenocarcinoma [2]. Moreover, the CHECKMATE-036 trial had previously demonstrated the efficacy of nivolumab plus ipilimumab (anti-CTL4), whose combination was associated with meaningful antitumoral activity, durable response, encouraging long-term overall survival (OS) and satisfactory tolerance as second-line treatment for patients with chemotherapy-refractory esophagogastric cancer [3].
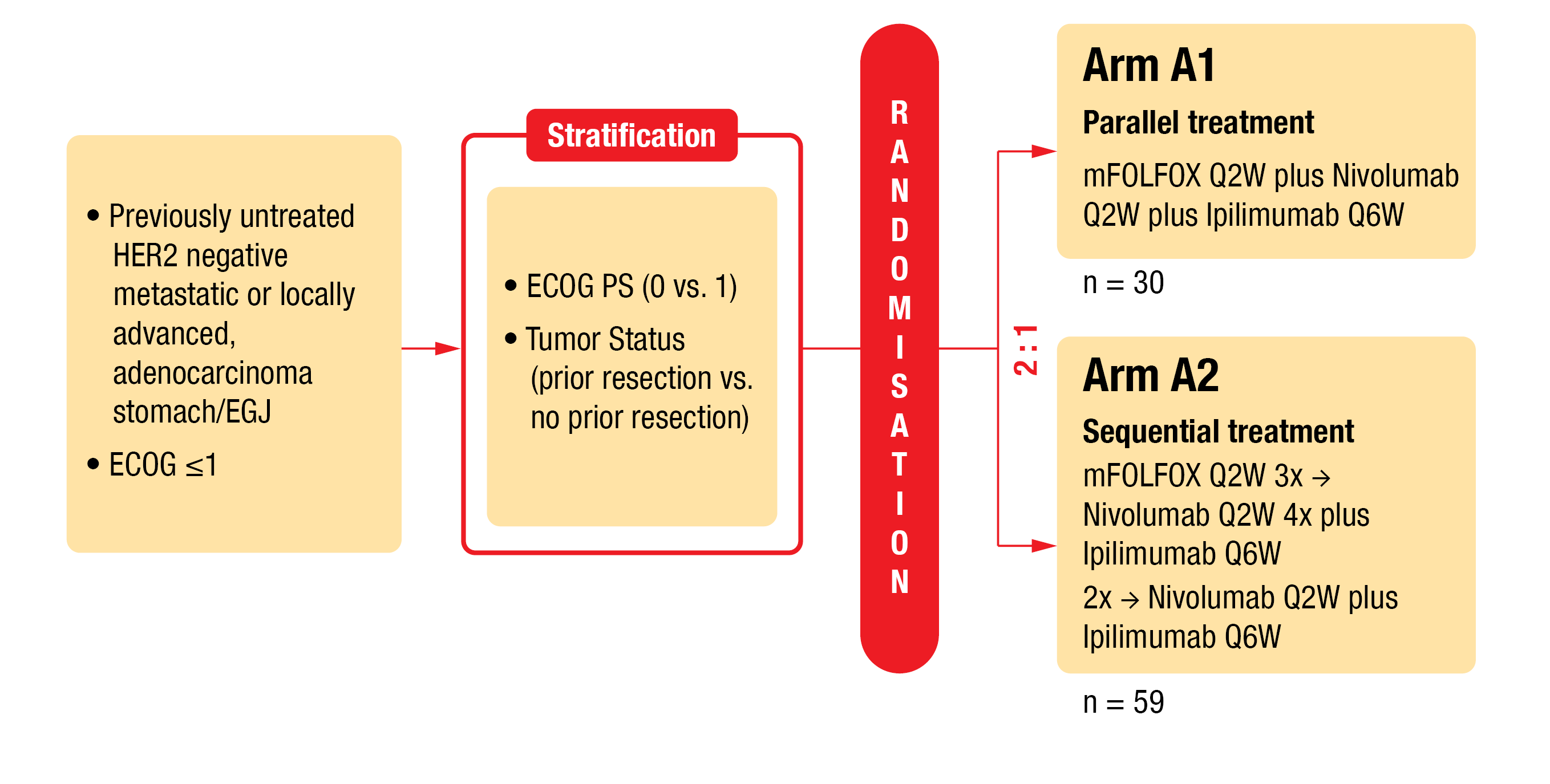
In the AIO-STO-0417 (Moonlight) trial, a four-arm phase II trial (NCT03647969), the combination of modified FOLFOX (mFOLFOX) chemotherapy plus nivolumab and ipilimumab (arm A) versus mFOLFOX alone (arm B) was evaluated as first-line therapy for patients with GC/GEJA [4]. To reduce toxicity, there is a need for an alternative treatment. Thus, in a second part of the Moonlight study (Figure 1), short-term induction chemotherapy is under investigation. In this study, mFOLFOX plus nivolumab and ipilimumab (arm A1) versus induction therapy followed by nivolumab and ipilimumab (arm A2) were assessed for toxicity and efficacy. Outcomes were presented by Lorenzen et al. at ESMO 2022 [5].
Patients with previously untreated HER2 (human epidermal growth factor receptor 2) negative metastatic or locally advanced gastric/gastroesophageal junction adenocarcinoma (G/GEJA) were stratified according to ECOG (0 or 1) and tumor status (prior resection or not). Eligible patients were randomized (1:2) to receive either parallel treament (arm A1: mFOLFOX every second week [Q2W] plus nivolumab [240 mg, Q2W] plus ipilimumab [1 mg/kg, Q6W]) or sequential treatment (arm A2: 3 cycles of mFOLFOX induction treatment Q2W, followed by immunotherapy consisting of 4 administrations of nivolumab [240 mg, Q2W] and 2 administrations of ipilimumab [1 mg/kg, Q6W]). The primary endpoint was progression-free survival (PFS) at six months, while key secondary endpoints were OS, PFS, overall response rate (ORR) and safety.
The parallel treatment was administered to 30 patients for a median of 11.5 cycles, while 60 patients received the sequential treatment for a median of eight cycles. At the time of data cutoff (July 22, 2022), the median follow-up was 9.3 months. The median age was 59.5 years in arm A1 and 63.5 years in arm A2. In total, 41 % of patients had a PD-L1 combined positive score (CPS) ≥ 1 (available in 74 % of patients). Progression-free survival at six months was twice as high in the parallel treatment arm compared to the sequential treatment arm (6-month PFS: 60 % in arm A1 vs 30 % in arm A2). The median PFS was longer in arm A1 than in arm A2 (7.29 vs 3.98 months; log rank p=0.0261), as well as the median OS (10.12 vs 7.85 months; log rank p=0.3604). Of note, a similar advantage was observed in the PD-L1 positive subpopulation for the parallel treatment (median OS, 16.46 months in arm A1 vs 6.87 months in arm A2; log rank p=0.4512). When comparing arm A1 and arm A2, a higher proportion of patients obtained a response (ORR, 46.7 % [10.0 % CR, 36.7 % PR] vs 30.0 % [6.7 % CR, 23.3 % PR], respectively). Moreover, the obtained responses were more durable in the parallel treatment arm than in the sequential one (8.4 vs 4.3 months).
However, patients in arm A1 experienced more grade ≥ 3 treatment-related adverse events (TRAEs) than those in arm A2 (70.0 % vs 43.3 %). The most common any-grade TRAEs across both arms were diarrhea, fatigue, nausea, decreased neutrophil count and peripheral sensory neuropathy. There was one treatment-related death in both groups.
Although associated with better tolerability, FOLFOX induction followed by nivolumab plus ipilimumab was less effective than FOLFOX plus nivolumab and ipilimumab. Thus, this study does not support the use of sequential treatment in the first-line setting. However, the results must be interpreted with caution due to the small number of participants and a low PD-L1 expression rate in both treatment arms.
Figure 1: Design of the AIO-STO-0417 (Moonlight) trial (arms A1 and A2).
PRODIGE 59 – DURIGAST: 2L FOLFIRI plus ICI(s)
Although the combination of ICIs with chemotherapy has demonstrated its efficacy as first-line treatment of advanced G/GEJA [2], treatment options in second line remain limited and the current standard-of-care is still based on chemotherapy (paclitaxel, ramucirumab or irinotecan as monotherapy or in combination with 5-FU). Tougeron et al. presented the results of a first 2L study combining FOLFIRI (leucovorin, 5-FU and irinotecan) with one or two ICIs at this year’s ESMO meeting [6].
PRODIGE 59 – DURIGAST is a French, randomized, non-comparative, phase II trial (NCT03959293) assessing the efficacy and safety of the combination of FOLFIRI plus durvalumab (anti-PD-L1) with or without tremelimumab (anti-CTLA-4) as 2L treatment of advanced G/GEJA. Platinum-based first-line chemotherapy pretreated patients with G/GEJA, who were ICI-naive and had a good performance status (ECOG 0 or 1) were equally randomized (1:1) to receive either FOLFIRI (intravenously [IV], Q2W) plus durvalumab (1500 mg, IV, Q4W) or FOLFIRI plus durvalumab plus tremelimumab (75 mg, IV, Q4W, 4 cycles only) until disease progression. The primary endpoint was PFS at four months.
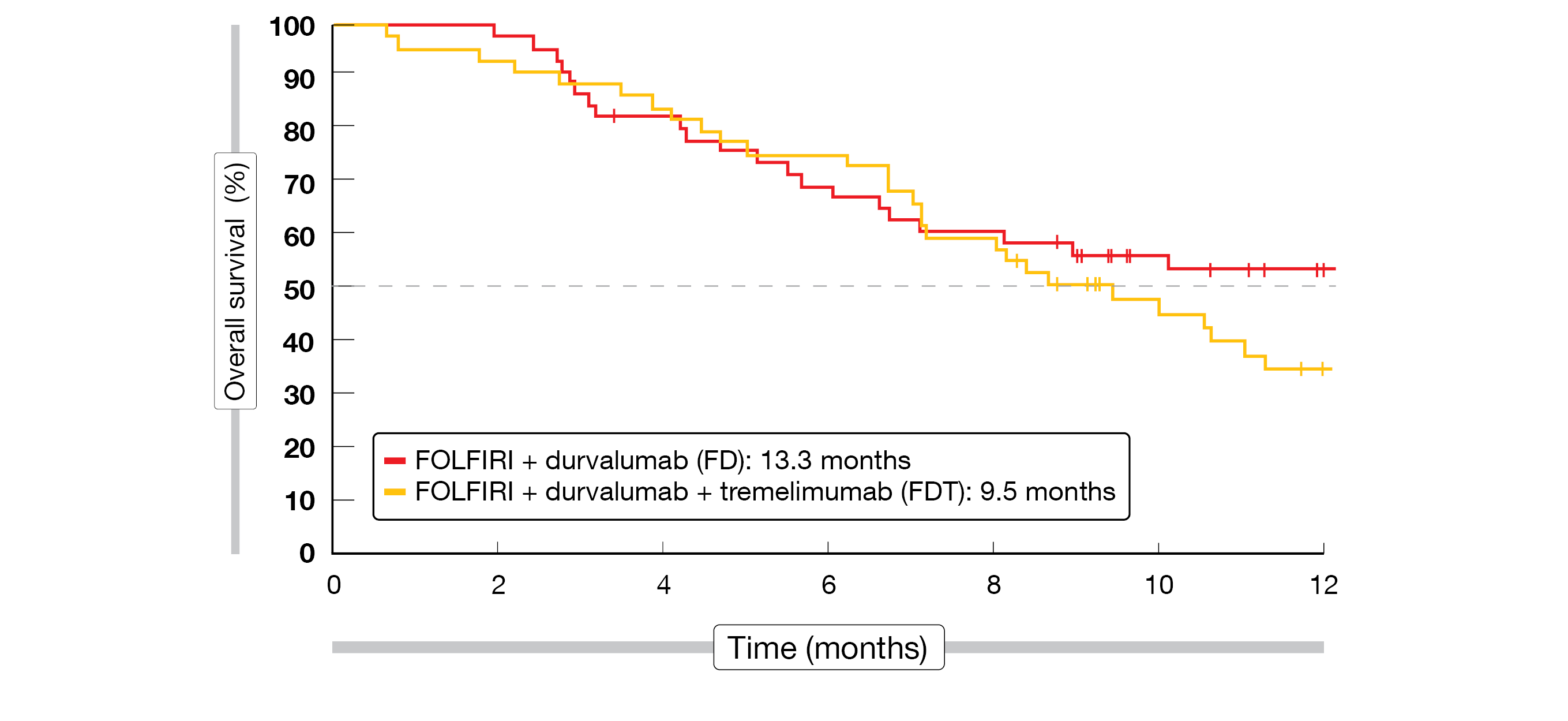
In total, 92 patients were enrolled in this trial between August 2020 and June 2021. Most patients had GEJA (53.3 %) with intestinal subtype (50 %) and synchronous delay of metastatic disease (65.2 %), with the liver being the most frequent metastatic site (40.2 %). At the time of analysis, six patients receiving FOLFIRI plus durvalumab (FD) and twelve patients receiving FOLFIRI plus both ICIs (FDT) were still under treatment. The primary endpoint was not met, as the 4-month PFS was inferior to 70 % in both groups (44.7 % in FD and 55.6 % in FDT). There was no advantage of the combination of FD versus FDT in terms of either OS (13.3 vs 9.5 months, Figure 2) or disease control rate (DCR, 67.4 % vs 68.9 %). To note, a larger number of patients had a controlled disease at twelve months in the FDT arm (15.7 %, n = 7) as compared to the FD arm (4.3 %, n = 2); Moreover, about 30 % of the patients in both groups showed OS longer than twelve months.
The safety profile of combining one or two ICIs to FOLFIRI was acceptable, with an equal proportion of grade ≥3 TRAEs (47.8 %). The most frequent grade ≥3 TRAEs were decreased neutrophil count (15.2 %) and vomiting (6.5 %) in the FD treatment group and decreased neutrophil count (23.9 %) and diarrhea (10.9 %) in the FDT treatment group.
Although the combination of FOLFIRI plus durvalumab or durvalumab plus tremelimumab did not meet its primary endpoint, both arms demonstrated a clinically relevant PFS, and an increased OS compared to historic data with chemotherapy alone. As the efficacy of both combinations seems promising in specific subgroups, predictive markers of efficacy (PD-L1 status, immune scores, tumor mutation burden and microbiota) are currently under investigation in ancillary studies.
Figure 2: Overall survival in the PRODIGE 59 – DURIGAST trial
DESTINY-Gastric02: 2L T-DXd in patients with HER2+ G/GEJA
Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate consisting of a humanized HER2 targeting antibody with the same amino acid sequence as trastuzumab, a cleavable tetrapeptide-based linker, and DXd, a cytotoxic topoisomerase I inhibitor, as its released payload [7]. This targeted therapy was approved by the FDA for the treatment of locally advanced or metastatic HER2-positive G/GEJA, who previously received a trastuzumab-based regimen [8]. At this year’s ESMO meeting, Ku et al. presented the updated analysis of DESTINY-Gastric02, an open-label, phase II study (NCT04014075) conducted in Western patients [9].
Eligible patients had a pathologically documented, unresectable or metastatic G/GEJA, a centrally confirmed HER2-positive disease (defined as immunohistochemistry (IHC) 3+ or IHC 2+/in situ hybridization (ISH)+) from a biopsy performed after progression on trastuzumab-based first-line regimen, and a good performance status (ECOG 0 or 1). All patients enrolled in this single-arm study received T-DXd (6.4 mg/kg, Q3W). The primary endpoint was confirmed ORR (cORR) as assessed by independent central review (ICR); the ICR-confirmed PFS, OS, duration of response (DoR), safety and patient-reported outcomes (PROs) based on health-related quality of life (HRQoL) were secondarily analyzed.
A total of 79 patients were included in this study. After a median follow-up of 10.2 months (data cut-off: November 8, 2021), a cORR of 41.8 % (5.1 % of patients with a complete response [CR], 36.7 % of them with a partial response [PR]) was reported. Confirmed DCR (81 %; 95 % CI: 70.6-89.0), median DoR (8.1 months; 95 % CI: 5.9-NE) and median time to response (TTR, 1.4 months; 95 % CI, 1.4-2.7) were similar to the results from the primary analysis (median follow-up of 5.9 months). The reported median OS was 12.1 months (95 % CI, 9.4-15.4) and the median PFS reached 5.6 months (95 % CI, 4.2-8.3).
The updated safety data were generally consistent with the established T-DXd safety profile [10]. Overall, 55.7 % of patients experienced a grade ≥3 treatment-emergent adverse event (TEAE), the most common TEAEs being nausea (67.1 %), vomiting (44.3 %), and fatigue (41.8 %). A total of eight patients experienced an adjudicated drug-related interstitial lung disease (ILD)/pneumonitis (grade 1: 2.5 %, grade 2: 5.0 % and fatal grade 5: 2.5 %). Median time to onset of adjudicated drug related ILD/pneumonitis was 80.5 days, with a median duration of 36.0 days. The HRQoL was maintained during treatment with T-DXd from baseline throughout to cycle 7 without any worsening.
After seven months of additional follow-up, T-DXd continues to demonstrate a clinical benefit and a tolerable safety profile as well as maintained HRQoL as second-line treatment for Western patients with HER2-positive unresectable/metastatic G/GEJ cancer.
1L KN026/KN046 dual therapy in HER2+ G/GEJ
At ESMO 2022, Gong et al. presented the preliminary analysis of a phase II trial (NCT04521179) assessing the safety and the efficacy of a dual therapy in HER2-positive patients with locally advanced unresectable or metastatic gastric/gastroesophageal junction (G/GEJ) cancer without prior systemic treatment. This treatment combines two novel bispecific antibodies: KN026, an anti-HER2 antibody that binds simultaneously to two non-overlapping epitopes of HER2 and thus leading to a dual HER2 signal blockade; and KN046, a bispecific antibody preventing both the interaction of PD-L1 with PD-1 and CTLA-4 with CD80/CD86 [11].
Eligible patients received KN026 (30 mg/kg, Q3W on Cycle 1/Day 1 & Cycle 1/Day 8) combined with KN046 (5 mg/kg, Q3W) until disease progression or intolerable toxicity. The co-primary endpoints are ORR and DoR according to RECIST v1.1. Additionally, PFS, clinical benefit rate (CBR) and OS, are analyzed secondarily. Since January 30, 2022, a total of 31 patients (median age, 64 years) were enrolled so far, with 26 patients still receiving study treatment. HER2 status was determined with HER2 gene amplification in all patients (83.9 % had IHC3+ and 16.1 % IHC2+). Most patients (80.6 %) presented with ECOG 1; most of them had distant lung (61.3 %) or liver (12.9 %) metastases at enrollment.
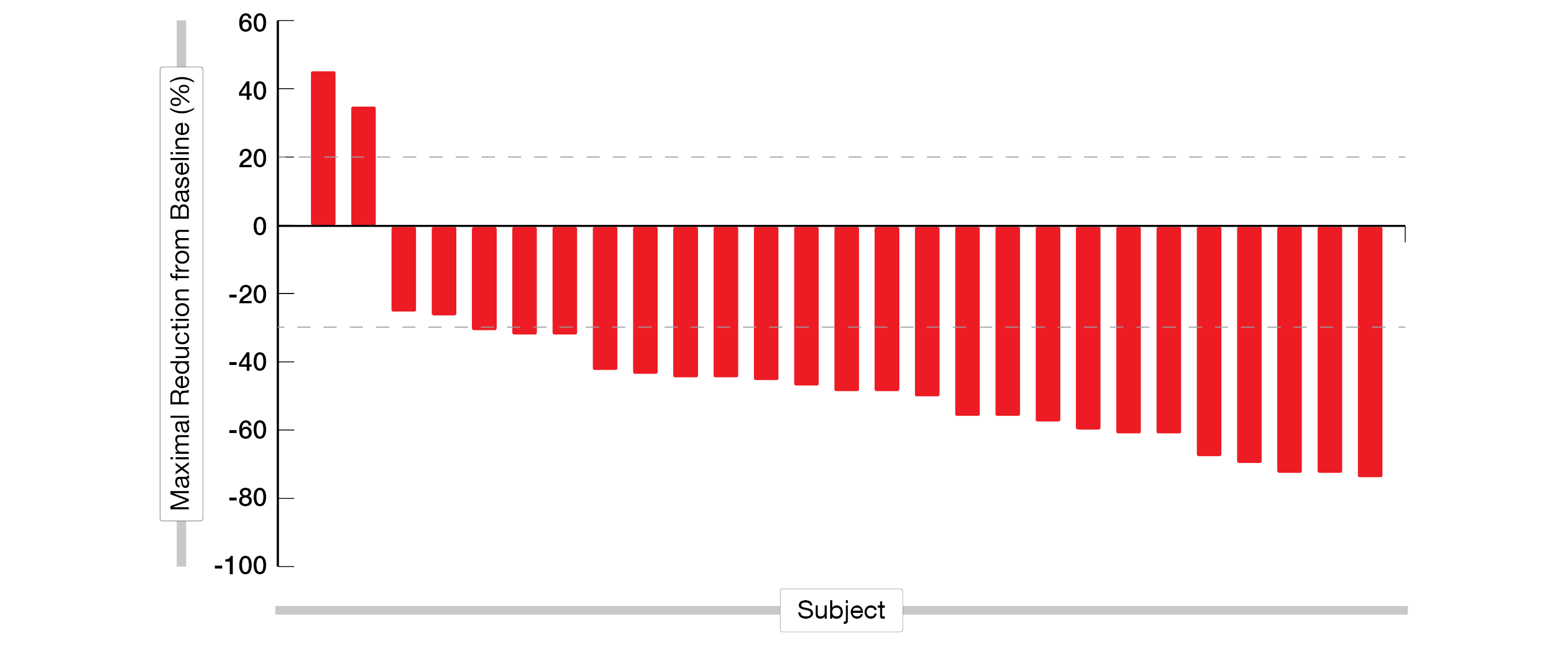
Overall, 27 patients were evaluable for efficacy at the time of the preliminary analysis with twelve confirmed PRs, nine unconfirmed PRs, four stable disease (SD) and two progressive disease (PD) (Figure 3). The ORR was 77.8 %, while 92.6 % of the patients had a controlled disease (DCR).
Considering safety, 80.6 % experienced at least one TRAE, most frequently being diarrhea (32.3 %), pyrexia (32.3 %) and leukopenia (22.6 %). Most TRAES were mild to moderate; however, grade ≥3 TRAES occurred in 16.1 %, with diarrhea (6.5 %) and pyrexia (3.2 %) being the most commonly reported ones and almost all patients recovered. However, three patients discontinued their treatment following KN046-treatment related AEs, while there was no treatment discontinuation related to KN026 treatment. No treatment-related death was reported.
Although preliminary, these clinical data demonstrate a synergistic antitumoral activity and a manageable safety of KN026 combined with KN046 treatment in HER2-positive G/GEJ cancer systemic therapy-naïve patients. Further studies are warranted to confirm these outcomes.
Figure 3: Waterfall plot of individual patient response to first-line KN026 plus KN046 treatment in advanced HER2 positive G/GEJ cancer.
First-in-human trial of TST001
Targeted therapy and immunotherapy have revolutionized treatment of various cancers in the past decade, and are currently developed to identify ideal monoclonal antibodies specific to tumor proteins only. One of these investigated proteins is claudin 18.2 (CLDN18.2), which is involved in tumor development, as well as in cancer progression [12]. TST001 is a recombinant humanized antibody presenting a high affinity to CLDN18.2, that can eliminate cancer cells via antibody-dependent cytotoxicity and complement dependent cytotoxicity. In combination with chemotherapy, TST001 has already shown synergistic effects in preclinical studies [13].
At this year’s ESMO meeting, Shen et al. reported on an updated analysis of the open label, phase I, first-in-human trial of TST001 (NCT04495296) [14]. In this dose escalation and expansion study safety, tolerability, and preliminary efficacy of TST001 combined with capecitabine and oxaliplatin (CAPOX) as first-line treatment in locally advanced or metastatic G/GEJ cancer are evaluated. Patients recruited in the dose escalation phase had no prior systemic treatments and undetermined CLDN18.2 expression level. However, a positive CLDN18.2 expression confirmed by a central laboratory was required to be enrolled in the dose expansion phase of this trial.
As of August 4, 2022, 51 patients had been enrolled and dosed with TST001 (1, 3, 6 or 8 mg/kg) plus CAPOX Q3W, following a 3+3 design. After the dose escalation phase, the regimen of the expansion phase was determined as 6 mg/kg TST001 plus CAPOX Q3W with which 36 patients were treated. Median follow-up was 65 days on average (median age, 56 years). Most patient had GC (92.2 %) and 1-3 metastases. No patients experienced dose limiting toxicity and 36 patients were still on treatment at the time of the presentation.
TST001 combined with CAPOX was well tolerated; 89.7 % of patients treated with 6 mg/kg experienced any grade TEAEs and 17.9 % grade ≥ 3 TEAEs with hypertension (5.1 %) being the most common one. No patient had to discontinue treatment due to TEAEs, however, twelve patients experienced dose delay, five a dose reduction and eleven a dose interruption. One patient died after treatment discontinuation for unknown reason. According to pharmacokinetics analysis, the elimination half-life of TST001 was four to seven days, regardless of the administrated dose.
Amongst the 15 patients in the expansion phase with a measurable disease and at least one tumor assessment, 73.3 % had a partial response (PR) and 26.7 % a stable disease (SD). Disease control rate was 100 %.
This interim analysis confirmed the safety of the novel agent TST001 in combination with CAPOX as first-line treatment for G/GEJ cancer. Efficacy data of this combination were also encouraging, although a correlation of antitumor activity with CLDN18.2 expression thresholds needs to be evaluated. TST001 is currently investigated in other combinations and in various indications (NCT05190575, NCT04495296) and a phase III trial is under consideration.
PANDA trial: neoadjuvant atezolizumab in HER2+ G/GEJA
The standard-of-care for non-metastatic, resectable G/GEJA currently consists of perioperative docetaxel-based triplet FLOT (5-FU plus leucovorin, oxaliplatin and docetaxel) chemotherapy [15]. This therapy is associated with a 16 % pathological complete response (pCR) and a 37 % major (complete plus subtotal) pathologic response (MPR) [16]. The clinical efficacy of anti–PD-(L)1 drugs has already been established in advanced unresectable tumors and supports the evaluation of immunotherapy against earlier disease stages [17].
The PANDA study presented at ESMO 2022 by Chalabi et al. is a single-center, phase II trial (NCT03448835) exploring the safety and the feasibility of atezolizumab (anti-PD-L1) based neo-adjuvant therapy in non-metastatic, primary resectable G/GEJA treatment-naïve patients [18]. The investigational treatment consists of one cycle of atezolizumab monotherapy (1200 mg) followed by four cycles of atezolizumab plus docetaxel-oxaliplatin-capecitabin (A-DOC), followed by surgery seven weeks after the last A-DOC cycle. Baseline tumor-staging was assessed via an oesophagoduodenoscopy, biopsies, a CT scan or/and FDG-PET-CT for all patients; an ultrasound endoscopy in GEJA patients or a diagnostic laparoscopy in diffuse type G cancers was additionally performed to exclude metastatic conditions. A radiological (via CT scan or/and FDG-PET-CT) post-treatment staging was also done prior to the last cycle of treatment. Tumor samples were collected at baseline, after atezolizumab monotherapy, after first A-DOC and at resection. The primary endpoint was safety and feasibility. Secondary endpoints were disease-free survival (DFS), pCR, MPR and translational analyses (immunohistochemistry [IHC], as well as DNA and RNA sequencing).
Twenty patients (median age, 62 years) were enrolled and followed-up over a median of 29 months. Patients’ clinical stage ranged from N0 to N3 (N0, 25 %; N1, 50 %; N2, 20 %; N3, 5 %). At the time of data cut-off (June 15, 2022), 75 % of patients were alive and disease-free. In almost half of the patients, tumor and lymph node regression was reported (pCR, 45 %; 95 % CI, 23-68). In total, a MPR (≤10 % residual vital tumor) was attained in 70 % (95 % CI, 46-88). At the time of data cut-off, disease recurrence occurred only in non-responders (NR) presenting with more than 50 % residual vital tumor (30 %; 95 % CI, 12-54), after a median time of ten months post-surgery.
Overall, 10 % of patients had immune-related grade 3 adverse events (irAEs), consisting of headache, hepatitis, and diarrhea. Chemotherapy-related grade 3 AEs were experienced by 20 %, including febrile neutropenia (n = 3) and diarrhea (n = 1), while 50 % of patients had surgery-related grade 3 AEs, to the same extent as historical data [15, 19].
Besides efficacy and safety evaluation, biomarker analysis highlighted differences between responders and non-responders. Following atezolizumab monotherapy, responders presented with increased transcription of immunological markers such as IFNγ, CD8, CXCL1, PD-L1 and PD-1. Moreover, according to baseline IHC analysis, there was a significantly higher CD8/PD-1-positive T cell infiltration in responders compared to non-responders. There was no difference between the two groups regarding the tumor mutational burden.
These preliminary results of the PANDA trial, showing promising pathologic responses in G/GEJ cancer highlight the efficacy of neoadjuvant atezolizumab plus chemotherapy in this patient cohort and suggest promising biomarker tools to predict response to treatment.
REFERENCES
- Sung, H, et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249.
- Janjigian, YY, et al., First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398(10294): 27-40.
- Janjigian, YY, et al., CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol 2018; 36(28): 2836-2844.
- Al-Batran, SE, et al., Modified FOLFOX versus modified FOLFOX plus nivolumab and ipilimumab in patients with previously untreated advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction: Moonlight, a randomized phase 2 trial of the German Gastric Group of the AIO. J Clin Oncol 2019; 37(suppl 15; Abstr TPS4144).
- Lorenzen, S, et al., FOLFOX plus nivolumab and ipilimumab versus FOLFOX induction followed by nivolumab and ipilimumab in patients with previously untreated advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction – results from the randomized phase 2 Moonlight trial of the AIO-ST-047. Ann Oncol 2022; 33(suppl 7; Abstr 1203O).
- Tougeron, D, et al., 1204MO – PRODIGE 59 – DURIGAST trial: A randomised phase II study evaluating FOLFIRI plus durvalumab and FOLFIRI plus durvalumab plus tremelimumab in second-line treatment of patients with advanced gastric or gastro-oesophageal junction adenocarcinoma. Ann Oncol 2022; 33(suppl 7; Abstr 1204MO).
- Shitara, K, et al., Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020; 382(25): 2419-2430.
- FDA approves fam-trastuzumab deruxtecan-
nxki for HER2-positive gastric adenocarcinomas. 2021, last accessed October 03, 2022; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-positive-gastric-adenocarcinomas. - Ku, G, et al., Updated analysis of DESTINY-Gastric02: A phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable/metastatic gastric/gastroesophageal junction (GEJ) cancer who progressed on or after trastuzumab-containing regimen. Ann Oncol 2022; 33(suppl 7; Abstr 1205MO).
- Van Cutsem E., et al., Primary analysis of a phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable or metastatic gastric or gastroesophageal junction (GEJ) cancer who progressed on or after a trastuzumab-containing regimen. Ann Oncol 2021; 32(suppl 5; Abstr LBA55).
- Gong, J, et al., The preliminary efficacy and safety of KNO26 combined with KN046 treatment in HER2-positive locally advanced unresectable or metastatic gastric/gastroesophageal junction cancer without prior systemic treatment. Ann Oncol 2022; 40(suppl 4; Abstr 1210P).
- Singh, P, et al., Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol 2017; 10(1): 105.
- Gabrail, NY, et al., A phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of TST001 in patients with locally advanced or metastatic solid tumors. J Clin Oncol 2022; 40(suppl 4; Abstr TPS375).
- Shen, L, et al., Updated report of a phase I study of TST001, a humanized anti-CLDN18.2 monoclonal antibody, in combination with capecitabine and oxaliplatin (CAPOX) as a first-line treatment of advanced G/GEJ cancer. Ann Oncol 2022; 33(suppl 7; Abstr 1254P).
- van der Werf, LR, et al., Reporting National Outcomes After Esophagectomy and Gastrectomy According to the Esophageal Complications Consensus Group (ECCG). Ann Surg 2020; 271(6): 1095-1101.
- Al-Batran, SE, et al., Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016; 17(12): 1697-1708.
- Topalian, SL, et al., Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020; 367(6477).
- Chalabi, M, et al., Neoadjuvant atezolizumab plus chemotherapy in gastric and gastroesophageal junction (G/GEJ) adenocarcinoma: The PANDA study. J Clin Oncol 2022; 33(suppl 7; Abstr 1219).
- Al-Batran, SE, et al., Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019; 393(10184): 1948-1957.
© 2022 Springer-Verlag GmbH, Impressum