Long-term results and other findings from clinical trials
MURANO: 7-year data and retreatment substudy
Fixed-duration venetoclax plus rituximab (VenR; n = 194) was tested against bendamustine plus rituximab (BR) for six months (n = 195) in the global, open-label, randomized phase III MURANO study that enrolled patients with relapsed/refractory CLL. In the experimental arm, 6 cycles of rituximab were administered, and venetoclax monotherapy was taken for a total of 24 months. Indeed, VenR gave rise to improvements in progression-free survival (PFS; 53.6 vs. 17.0 months; p < 0.0001) and overall survival (OS), with 5-year OS rates of 82.1 % vs. 62.2 % (p < 0.0001) [1].
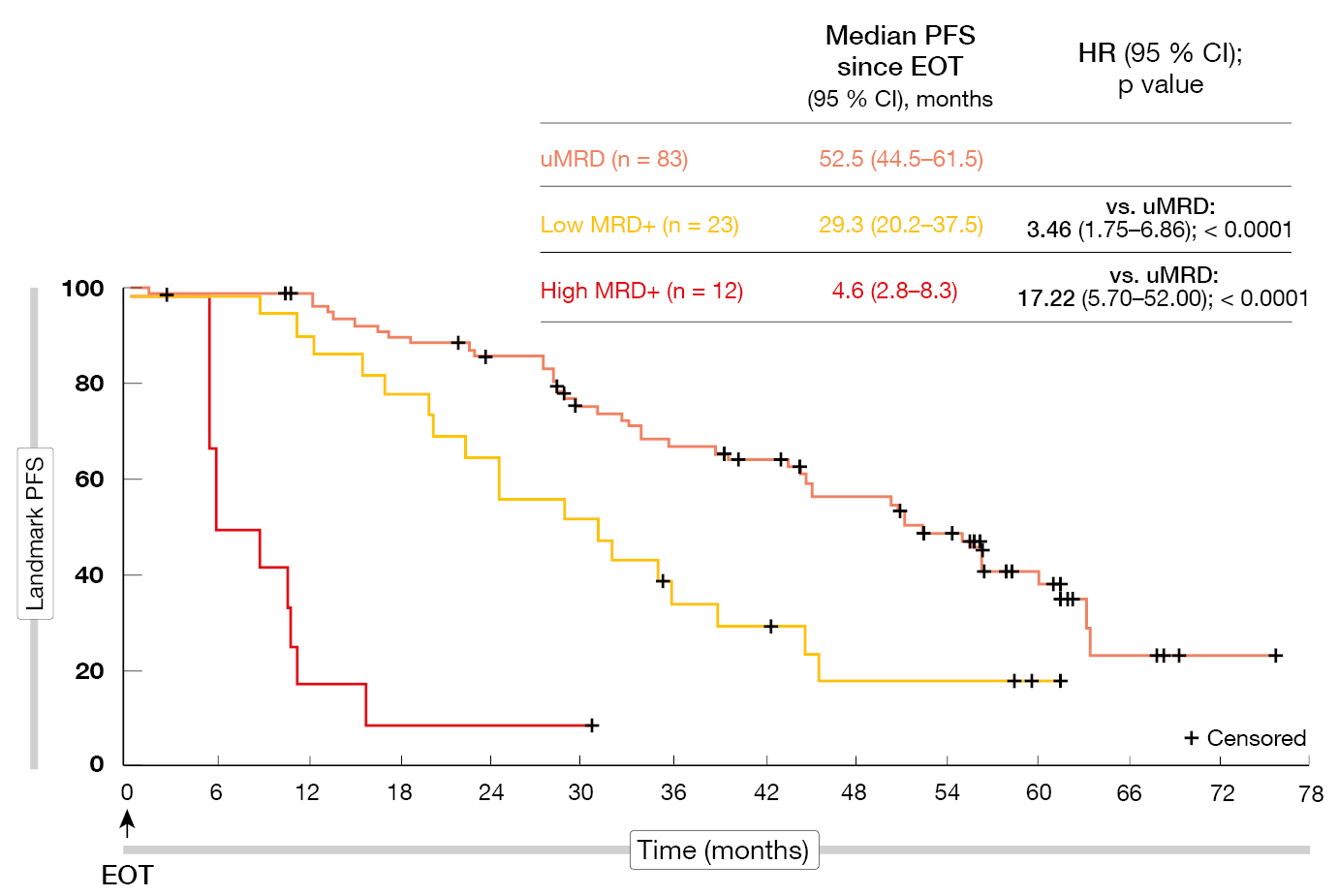
According to the final analyses after a median follow-up of approximately seven years, the benefits of VenR over BR were sustained [2]. Median PFS was 54.7 vs. 17.0 months (HR, 0.23; p < 0.0001), with the 7-year PFS rates being 23.0 % and not estimable, respectively. Median OS had still not been achieved in the experimental arm and was 87.8 months in the control arm (HR, 0.53; p < 0.0002). At 7 years, 69.6 % vs. 51.0 % of patients were alive. Time to the next anti-leukemic treatment was longer for VenR (63.0 vs 24.0 months; HR, 0.30). Most patients who received the full two years of VenR treatment had undetectable MRD (uMRD) at the end of therapy (EOT). Patients with uMRD showed significantly longer PFS from EOT than those with low MRD positivity (HR, 3.46; p < 0.0001) and high MRD positivity (HR, 17.22; p < 0.0001; Figure 1). For OS, these differences were not significant. Favorable baseline characteristics such as TP53 wild-type and IGHV mutation were over-represented in the group with enduring uMRD. If MRD conversion with subsequent disease progression occurred, it did not do so until approximately four years after EOT. No new safety signals had been identified since the 5-year data cutoff.
An amendment has been added to the initial study design that allows for retreatment of patients with disease progression on VenR in a substudy. Twenty-five of the 34 patients with disease progression who entered this substudy were retreated with VenR. Median time from the final drug dose in the main study to retreatment was 2.3 years. Most of these patients were classified as having high risk. In this population, median PFS from retreatment was 23.3 months, the best overall response rate was 72.0 %, the complete response (CR) rate was 24 %, and median OS had not been reached yet. Moreover, uMRD was still attainable in this high-risk patient group. Eight VenR-retreated patients achieved uMRD, although this was not sustained until the end of retreatment in any of these cases. The authors concluded that retreatment with VenR is a viable option for pretreated patients. Overall, these very mature data from the MURANO study continue to support the use of fixed-duration VenR in patients with relapsed/refractory CLL.
Figure 1: PFS according to MRD status in patients who completed two years of venetoclax treatment without disease progression
CLL2-BAAG
The phase II CLL2-BAAG trial was designed to investigate the combination of obinutuzumab, acalabrutinib and venetoclax in patients with relapsed/refractory CLL. After optional debulking with bendamustine, obinutuzumab was started in the first cycle, acalabrutinib in the second and venetoclax in the third. From cycle 4 onward, the patients received the triplet. Maintenance followed after the final restaging at cycle 6, and the treatment was continued until CR and uMRD were achieved. Forty-five patients were treated with the induction regimen, and 43 went on to receive maintenance. Among these, 25 patients were able to discontinue treatment after reaching CR and uMRD. Nine individuals completed the full 24 months of maintenance treatment. The group was enriched for risk factors such as unmutated IGHV, TP53 aberration, and complex karyotype. According to the primary endpoint analysis, all patients responded; after induction, 13 had already achieved CR [3]. Thirty-four patients (76 %) obtained uMRD.
Cramer et al. provided an update of the study after all patients had discontinued treatment [4]. These data confirmed that bendamustine, followed by obinutuzumab, acalabrutinib and venetoclax does not lead to cumulative or unexpected toxicity. Infections and cytopenias were the most common adverse events (AEs). Responses improved with continued maintenance. CR was achieved in 44 %, and the best uMRD rate was 93 %. The group of 25 patients who were able to stop treatment earlier due to obtaining CR and uMRD remained in uMRD for an extended period after EOT. At 30 months, the OS and PFS rates were 100 % and 88.2 %, respectively. To date, six cases of MRD conversion have emerged. Within the cohort of 21 patients who had been pretreated with targeted therapy including venetoclax (n = 10) before study inclusion, the results did not differ regarding the uMRD or PFS rates. However, this regimen is not ready for use in clinical routine practice, and further randomized trials exploring the triple combination are called for.
Update of the BOVen trial
In previously untreated patients with CLL/SLL, a multicenter, single-arm, phase II study has been initiated to assess MRD-driven duration of combined treatment with zanubrutinib, obinutuzumab, and venetoclax (i.e., the BOVen regimen). Indeed, the trial met its primary endpoint, with 89 % of patients reaching uMRD in both peripheral blood and bone marrow despite a median treatment duration of only 10 months that was due to the uMRD-driven treatment discontinuation design [5].
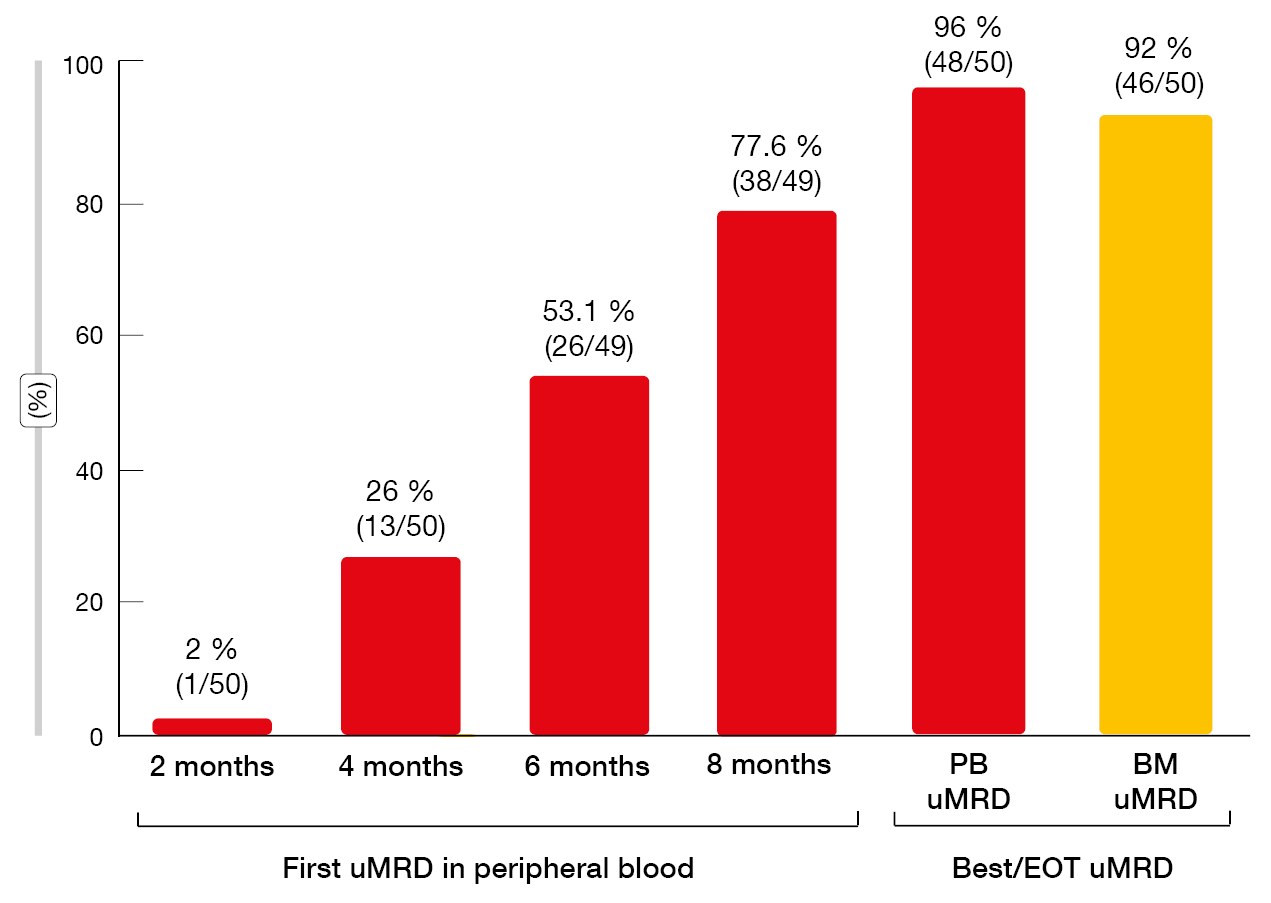
Study findings presented at iwCLL 2023 after an extended follow-up confirmed that the BOVen regimen led to frequent uMRD in the blood and bone marrow (96 % and 92 %, respectively; Figure 2) [6]. PFS was durable, with median PFS not having been reached in the total population (n = 50). Median MRD-free survival was 29.8 months after EOT in the group that showed uMRD in the bone marrow (n = 46). ΔMRD400, which was defined as a decrease in MRD in the peripheral blood at day 1 of cycle 5 (i.e., one month after reaching the target venetoclax dose), was demonstrated to be associated with longer MRD-free survival. In patients who achieved ΔMRD400 in addition to uMRD in the peripheral blood at EOT (n = 21), median MRD-free survival had not been reached, while this was 18.1 months in the group with uMRD at EOT but without ΔMRD400 (n = 13; p = 0.003). This benefit was achieved despite significantly shorter time on therapy in the group meeting the ΔMRD400 definition (8 vs. 13 months; p < 0.001). A phase II trial of BOVen investigating ΔMRD400-directed therapy in treatment-naïve patients with CLL/SLL is being planned based on the hypothesis that longer treatment duration for patients who fail to achieve ΔMRD400 will further improve uMRD duration.
Another trial in progress is BruVenG that is testing the addition of 6 cycles of obinutuzumab to zanubrutinib and venetoclax as consolidation in patients with detectable MRD after initial treatment with zanubrutinib/venetoclax [7]. The primary objectives are the MRD negativity rate after the induction phase and the MRD negativity rate after the end of triplet therapy. Patients who achieved MRD negativity on the initial doublet therapy are being observed.
Figure 2: MRD responses with zanubrutinib plus obinutuzumab and venetoclax
SEQUOIA: first-line zanubrutinib
The SEQUOIA study was initiated to explore the efficacy and safety of first-line zanubrutinib in CLL patients who are unsuitable for chemoimmunotherapy. Cohort 1 had no del(17p) and received either zanubrutinib (n = 241) or BR for 6 cycles (n = 238). Cohort 2 contained patients with del(17p) who were treated with zanubrutinib only (n = 111). At the interim analysis conducted after a median follow-up of 26.2 months, zanubrutinib showed superior PFS compared to BR (HR, 0.42; p < 0.0001), with similar results in the group with del(17p) [8]. At iwCLL 2023, Brown et al. reported updated findings after approximately 18 months of additional follow-up [9].
In cohort 1, median PFS had not been reached and was 42.2 months for zanubrutinib and BR, respectively (HR, 0.30; p < 0.0001). The estimated 42-month PFS rates were 82.4 % vs. 50.0 %. Significant PFS improvements on zanubrutinib treatment were noted for both patients with mutated IGHV (p = 0.00033) and unmutated IGHV (p < 0.0001). Median OS had not been reached in either group; the estimated 42-month OS rates were 89.4 % and 88.3 %, respectively. CR and CR with incomplete hematologic recovery (CRi) rates amounted to 17.4 % vs. 21.8 %. In cohort 2, median OS and PFS had not been reached, with estimated 42-month rates of 79.4 % and 89.5 %, respectively, and the CR/CRi rate was 14.5 %.
Zanubrutinib was well tolerated over the extended treatment period, and the AEs were in keeping with the known profile of BTK inhibitors. Atrial fibrillation events remained low over time, ranging from 1.3 % to 6.3 %. Overall, the results support the use of first-line zanubrutinib for elderly patients with CLL/SLL and those with del(17p).
HRQoL in ALPINE
In the relapsed/refractory setting, the randomized, open-label, phase III ALPINE trial was conducted to compare zanubrutinib (n = 327) with ibrutinib (n = 325). The final PFS analysis after a median follow-up of 29.6 months demonstrated superiority of zanubrutinib (HR, 0.65; p = 0.0024), which also applied to the overall response rate (86.2 % vs. 75.7 %; p = 0.0007) [10]. Health-related quality of life (HRQoL) was defined as a secondary endpoint of the ALPINE study and was reported at iwCLL 2023 [11].
According to this analysis, both agents gave rise to sustained improvements in global health scores and functioning scales from baseline to both cycle 7 and cycle 13. All improvements were clinically meaningful for the zanubrutinib arm. Given the generally good HRQoL at baseline in both arms, these differences were not significant. Decreases in fatigue and pain were observed for both zanubrutinib- and ibrutinib-treated patients, with the zanubrutinib arm experiencing clinically meaningful improvements in both symptoms at both cycles. More pronounced improvement was obtained for diarrhea with zanubrutinib, although this did not reach the predefined clinically meaningful threshold. The mean change from baseline in the EQ-VAS scores demonstrated a similar pattern of improvement with both therapies up to cycle 13. Long-term follow-up and additional analyses linking patient-reported outcome endpoints to clinical outcomes will further determine the effect of zanubrutinib on HRQoL.
Pooled safety data on zanubrutinib vs. ibrutinib
As is known, the use of the first-generation BTK inhibitor ibrutinib can be limited by AEs including cardiovascular and gastrointestinal toxicities that are attributed to off-target kinase inhibition [12-15]. The next-generation BTK inhibitor zanubrutinib has been designed to improve tolerability by maximizing BTK occupancy and minimizing off-target effects [16]. A previous pooled analysis of 779 patients from six clinical trials has demonstrated a consistent and generally well tolerated safety profile of zanubrutinib [17]. Brown et al. presented an updated pooled analysis comprising 1,550 patients with CLL/SLL and other B-cell malignancies from ten clinical studies at iwCLL 2023 [18]. These included the ASPEN and ALPINE trials that compared zanubrutinib head-to-head with ibrutinib. Median exposure was 34.4 months among all patients who received zanubrutinib, with 45.0 % being on treatment for ≥ 36 months.
Among non-hematologic treatment-emergent AEs (TEAEs) of zanubrutinib, upper respiratory tract infections and diarrhea were most common. No grade ≥ 3 TEAEs occurred in > 10 % of patients. In the pooled zanubrutinib population, deaths attributed to TEAEs occurred in 7.3 %; most (3.7 %) were due to infections, including COVID-19–related TEAEs. Cardiac-related TEAEs leading to death occurred less commonly with zanubrutinib than with ibrutinib (0.2 % vs 1.7 %) in the head-to-head trial populations.
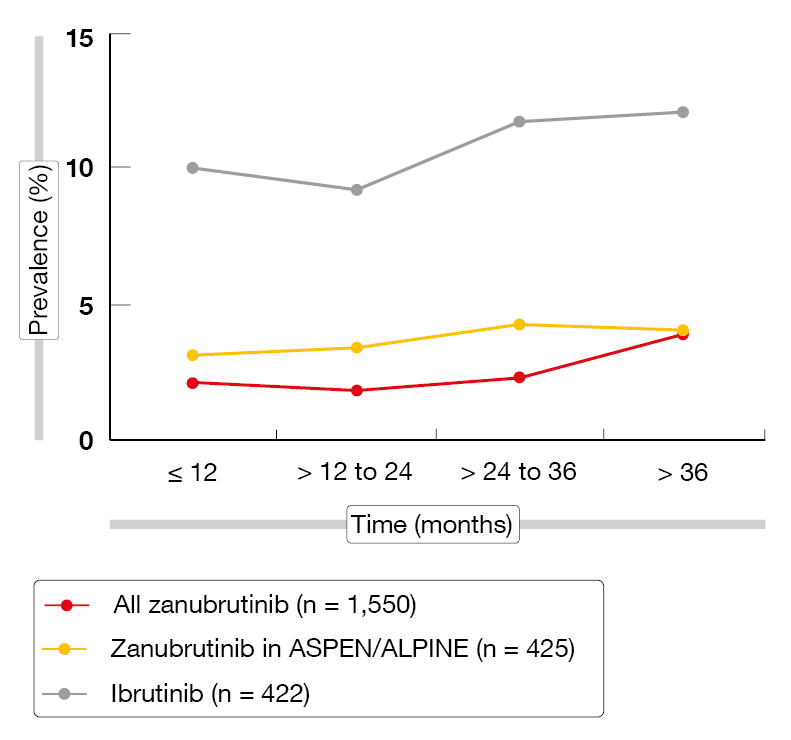
The prevalence of AEs of special interest (AESIs) tended to remain stable with zanubrutinib or to decrease over time. For atrial fibrillation, the rates were persistently lower than with ibrutinib (Figure 3). Exposure-adjusted incidence rates (EAIRs) of AESIs, including infections, were numerically lower with zanubrutinib than with ibrutinib in the ASPEN/ALPINE study populations, with the exception of neutropenia. Regarding hypertension, EAIRs were low and consistent for zanubrutinib across the studies except for ALPINE that was an outlier from the rates observed in other studies of zanubrutinib [10, 19]. Despite the higher hypertension rate in ALPINE, the EAIR for atrial fibrillation was significantly lower with zanubrutinib than with ibrutinib (p < 0.0001), which also applied to infections (p = 0.0098). These findings support zanubrutinib as an appropriate long-term treatment option for patients with B-cell malignancies.
Figure 3: Prevalence of atrial fibrillation/flutter over time with zanubrutinib vs. ibrutinib
Immune cell number changes in long-term treatment
Changes in T cells have been observed during long-term treatment with ibrutinib in CLL patients [20], although it is unclear to which extent these are induced by the inhibition of interleukin-2–inducible T cell kinase (ITK) rather than by the reduction of the tumor burden. Andersson et al. explored the impact of zanubrutinib treatment on T cell and natural killer (NK) cells in 16 patients with CLL who received long-term zanubrutinib therapy compared to ibrutinib. Eight of them had been included in the SEQUOIA trial and were treatment-naïve, while the other eight had been enrolled in the ALPINE study that assessed zanubrutinib in relapsed/refractory disease. After 24 months of follow-up, six patients in each study were still on treatment and had achieved partial response. All patients stayed on full-dose zanubrutinib during the whole treatment period.
According to the results presented at iwCLL 2023, changes in the T and NK cell profiles occurred in both treatment-naïve and relapsed/refractory patients and were similar to what had previously been observed in ibrutinib-treated patients [21]. Decreases were noted for CD19+ and CD8+ cells, as well as regulatory T cells and effector memory CD4+ and CD8+ cells, among others. Naïve CD4+ and CD8+ T-cells remained unchanged. NK cells decreased from week 16 and normalized at week 12 in relapsed/refractory patients while remaining stable in treatment-naïve patients. Taken together, these changes appear to be related to the reduction of the tumor burden rather than to ITK inhibition.
REFERENCES
- Seymour JF et al., Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood 2022; 140(8): 839-850
- Kater AP et al., Final 7-year follow-up and retreatment substudy analysis of MURANO: venetoclax-rituximab-treated patients with relapsed/refractory chronic lymphocytic leukemia. iwCLL 2023, poster 2002
- Cramer P et al., Obinutuzumab, acalabrutinib, and venetoclax, after an optional debulking with bendamustine in relapsed or refractory chronic lymphocytic leukemia (CLL2-BAAG): a multicentre, open-label, phase 2 trial. Lancet Haematol 2022; 9(10): e745-e755
- Cramer P et al., Bendamustine, followed by obinutuzumab, acalabrutinib and venetoclax in patients with relapsed/refractory chronic lymphocytic leukemia: updated results of the CLL2-BAAG trial of the German CLL Study Group. iwCLL 2023, session “Future directions for the treatment of relapsed/refractory CLL”, 8th October 2023, Boston, USA
- Soumerai JD et al., Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Haematol 2021; 8(12): E879-E890
- Soumerai J et al., Long-term follow-up of multicenter phase II trial of zanubrutinib, obinutuzumab, and venetoclax (BOVen) in previously untreated patients with chronic lymphocytic leukemia: impact of early MRD kinetics on posttreatment outcomes. iwCLL 2023, poster 2016
- Allan JN et al., Zanubrutinib and venetoclax as initial therapy for CLL with obinutuzumab triplet consolidation in patients with detectable minimal residual disease (BruVenG): trial in progress. iwCLL 2023, poster 3002
- Tam CS et al., Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol 2022; 23(8): 1031-1043
- Brown JR et al., Zanubrutinib vs bendamustine + rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma: extended follow-up of the SEQUOIA study. iwCLL 2023, poster 2010
- Brown JR et al., Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2023; 388(4): 319-332
- Brown JR et al., Zanubrutinib vs ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: impact on health-related quality of life. iwCLL 2023, poster 2013
- Coutre SE et al., Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv 2019; 3(12): 1799-1807
- O’Brien S et al., Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 2018; 18(10): 648-657
- Jain P et al., Ibrutinib with rituximab in first-line treatment of older patients with mantle cell lymphoma. J Clin Oncol 2021; 40(2): 202-212
- Buske C et al., Ibrutinib plus rituximab versus placebo plus rituximab for Waldenström’s macroglobulinemia: Final analysis from the randomized phase III iNNOVATE study. J Clin Oncol 2022; 40(1): 52-62
- Guo Y et al., Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem 2019; 62(17): 7923-7940
- Tam CS et al., Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv 2022; 6(4): 1296-1308
- Brown JR et al., Characterization of the safety/tolerability profile of zanubrutinib and comparison with the profile of ibrutinib in patients with B-cell malignancies: post hoc analysis of a large clinical trial safety database. iwCLL 2023, poster 2014
- Ma H et al., Rate of cardiac disorders in patients with B-cell malignancies who undergo treatment with Zanubrutinib. British Society for Haematology Annual Meeting 2023, abstract BSH23-OR19
- Mulder TA et al., Ibrutinib has time-dependent on- and off-target effects on plasma biomarkers and immune cells in chronic lymphocytic leukemia. Hemasphere 2021; 5(5): e564
- Andersson ML et al., Changes in immune cell numbers and profile during long-term zanubrutinib treatment in treatment-naïve and relapsed/refractory patients with chronic lymphocytic leukemia. iwCLL 2023, poster 2001
© 2023 Springer-Verlag GmbH, Impressum