Improved anti-EGFR strategies and other targeted innovations
FLAURA2: first-line osimertinib plus chemotherapy
In the first-line setting of EGFR-mutant advanced NSCLC, EGFR tyrosine kinase inhibitors (TKIs) such as the third-generation agent osimertinib have been established as the standard of care, although treatment is followed by disease progression in most patients. Data on the combined administration of EGFR TKIs with chemotherapy suggest enhanced efficacy [1-4]. These findings have prompted the implementation of the ongoing global, open-label, randomized FLAURA2 study to investigate osimertinib 80 mg OD plus pemetrexed and carboplatin or cisplatin (n = 279) compared to osimertinib monotherapy (n = 278) as first-line treatment of patients with advanced or metastatic NSCLC with activating EGFR mutations (i.e., either exon 19 deletion or L858R mutation). In the experimental arm, platinum-based treatments are being administered Q3W for four cycles and are followed by maintenance osimertinib plus pemetrexed Q3W. Stable central nervous system (CNS) metastases, which included untreated lesions, were allowed and were present in approximately 40 % of patients. Jänne et al. reported the results of the primary analysis of FLAURA2 at WCLC 2023 [5].
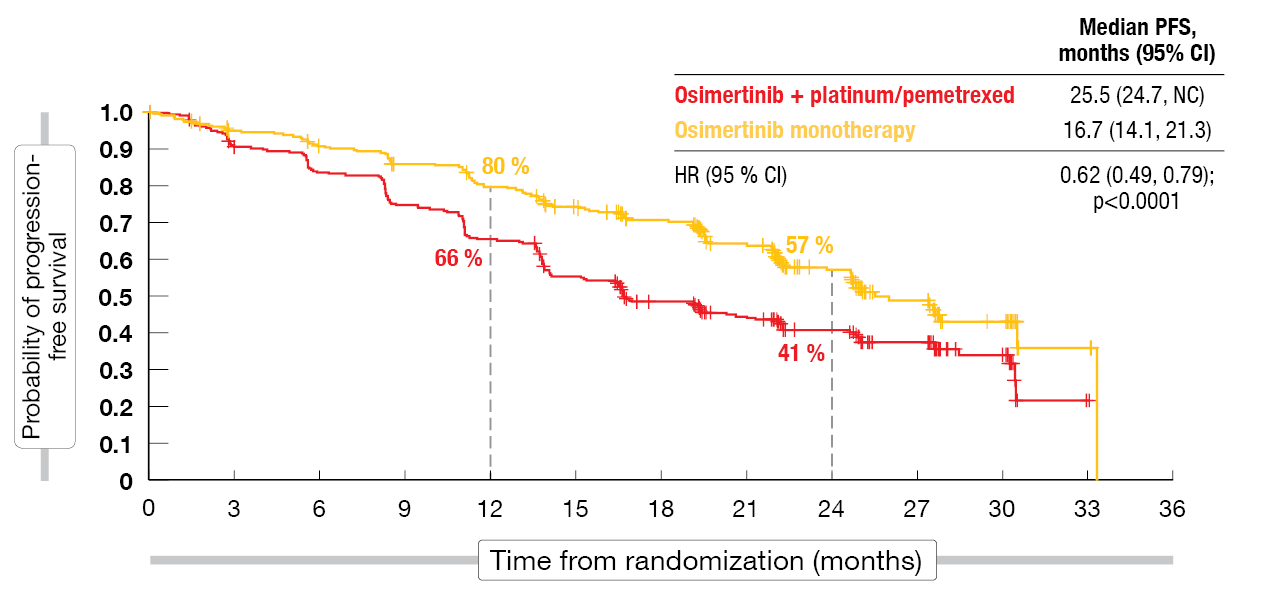
Progression-free survival (PFS) by investigator assessment, which was defined as the primary endpoint, significantly favored the osimertinib plus platinum/pemetrexed regimen. Median PFS was improved by 8.8 months (25.5 vs. 16.7 months), which translated into a 38 % reduction in the risk of progression and mortality (HR, 0.62; p < 0.0001; Figure 1). At 24 months, 57 % vs. 41 % of patients were progression-free. The analysis by blinded independent central review (BICR) confirmed this observation; here, the PFS difference reached 9.5 months (29.4 vs. 19.9 months; HR, 0.62; p = 0.0002).
Figure 1: Primary endpoint of FLAURA2: progression-free survival per investigator
Clinical benefits in subgroups
The PFS benefits were consistent across all pre-defined subgroups. Patients with CNS metastases at baseline derived greater PFS benefit with the combination relative to osimertinib monotherapy (24.9 vs. 13.8 months; HR, 0.47) than those without (27.6 vs. 21.0 months; HR, 0.75). Risk reductions of approximately 40 % resulted for patients with exon 19 deletion and L858R mutation alike. Second PFS (PFS2) and overall survival (OS) findings were immature at the time of the analysis. However, patients in the experimental arm showed longer PFS2 than those in the control arm (30.6 vs. 27.8 months; HR, 0.70; p = 0.0132). The objective response rates (ORRs) were 83 % vs. 76 %. All patients achieved median best percentage changes in target lesion size of approximately – 50 %, although the median duration of response was longer with the combination (24.0 vs. 15.3 months).
The safety profiles were as expected for each treatment and were manageable with standard medical procedures. While diarrhea was observed as the most common adverse event (AE) in the osimertinib monotherapy arm, the combined treatment most frequently gave rise to cytopenia, diarrhea and nausea. All grade 4 AEs in the osimertinib plus chemotherapy group were hematological toxicities. No common grade 4 AEs occurred in the monotherapy arm. Interstitial lung disease (ILD) was reported in 3 % vs. 4 % (all grades). Considering the statistically significant and clinically meaningful PFS improvement, osimertinib plus platinum/pemetrexed offers a new first-line treatment option for patients with advanced EGFR-mutated NSCLC. Ongoing analyses of the FLAURA2 trial will elucidate CNS response, patient-reported outcomes, post-progression endpoints, and ctDNA analyses.
Tepotinib plus osimertinib: INSIGHT 2
Mechanisms of resistance to first-line treatment with osimertinib are diverse, with almost half of them hitherto unidentified; for lack of other options, platinum-based chemotherapy is often administered after progression [6, 7]. A potential chemotherapy-sparing oral targeted approach is the combination of the MET inhibitor tepotinib with osimertinib, as MET amplification represents a common driver of secondary resistance to first-line osimertinib [8, 9]. Tepotinib 500 mg OD plus osimertinib 80 mg OD was tested in the open-label, phase II INSIGHT 2 study in patients who had advanced or metastatic EGFR-mutant NSCLC with acquired resistance to first-line osimertinib and MET amplification demonstrated by tissue-based FISH and/or next generation sequencing (NGS) based on liquid biopsy. The primary endpoint of INSIGHT 2 was the objective response by independent review in patients whose MET amplifications had been detected by tissue-based FISH.
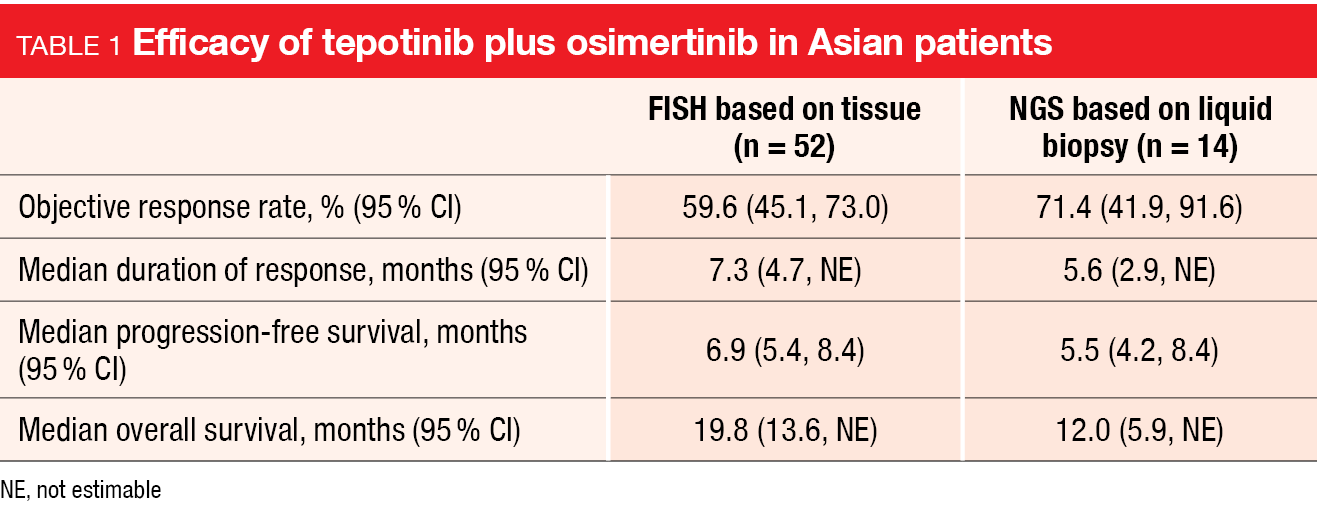
Overall, 128 individuals received tepotinib plus osimertinib. The cohort meeting the definition of the primary endpoint comprised 98 patients. In this group, the primary analysis reported by Kim et al. at WCLC 2023 after a minimum follow-up of 9 months showed an ORR of 50 % [10]. Responses were rapid and long-lasting, with a median duration of response of 8.5 months. The ORR was generally consistent across predefined subgroups. Median PFS and OS were 5.6 and 17.8 months, respectively. Similar outcomes resulted in the cohort that had MET amplification according to NGS (ORR, 54.8 %; median duration of response, 5.7 months; median PFS, 5.5 months; median OS, 13.7 months) and in the subgroup of Asian patients (Table 1).
In patients with brain lesions evaluable by RANO-BM who showed MET amplification determined by FISH (n = 24), the intracranial ORR was 29.2 %, with a complete response rate of 25.0 %. Intracranial disease control was achieved in 79.2 %, and intracranial PFS was 7.8 months. Systemic outcomes for patients with brain metastases were similar to those observed in the overall population. Baseline biomarker profiles were available for 69 patients. Here, the analysis demonstrated better outcomes in the absence of co-occurring mechanisms of osimertinib resistance.
Tepotinib plus osimertinib demonstrated a manageable safety profile. The most common any-grade AEs were diarrhea (49.2 %) and peripheral edema (40.6 %). Dose reductions due to treatment-related AEs (TRAEs) occurred in 20.3 %, and 10.2 % discontinued treatment due to TRAEs, which was most commonly pneumonitis (4.7 %). Four patients (3.1 %) had TRAEs leading to death that were considered potentially related to either trial drug (i.e., pneumonitis, respiratory failure after COVID-19 infection, and decreased platelet count). Health-related quality of life was maintained, with cough and pain improving on treatment. The safety profile observed among Asian patients was similar to the overall safety profile.
Update of CHRYSALIS-2
The single-arm CHRYSALIS-2 trial was conducted based on the assumption that the combination of EGFR/MET inhibition with platinum-based chemotherapy might address the diverse and polyclonal resistance after progression on osimertinib. Patients enrolled in this study had EGFR-mutant, advanced NSCLC after TKI treatment for a maximum of three prior lines. They received the EGFR-MET bispecific antibody amivantamab together with the third-generation EGFR TKI lazertinib and carboplatin/pemetrexed. While carboplatin was discontinued after four cycles, the other components of treatment were administered until progression.
The findings of the CHRYSALIS-2 trial presented at WCLC 2023 for 20 patients after a median follow-up of 13.1 months showed that the safety profile was consistent with that of the individual components [11]. Most AEs were grade 1 or 2. Ninety percent of patients developed neutropenia that was classified as grade ≥ 3 in 70 %, while thrombocytopenia emerged in 40 % (grade ≥ 3, 25 %). The highest rates for both types of cytopenia were seen in cycle 1, and grade ≥ 3 events decreased markedly after completion of carboplatin therapy. No cases of neutropenic fever occurred.
Fifty-five percent of patients remained on treatment at the time of the analysis. The ORR was 50 %, with eight of ten responders showing ongoing responses. The clinical benefit rate was estimated at 80 %. Among seven patients with disease stabilization, three had a stable disease duration of ≥ 6 months, and two remained on treatment. Median PFS in the total group was 14.0 months, while median OS had not been reached yet. At 12 months, 80 % of patients were alive, and 59 % were progression-free. As the authors noted, this suggests immune-driven durability of the treatment effect.
Overall, the combination of amivantamab, lazertinib and chemotherapy appeared promising and likely to address resistance after progression on osimertinib. Data from the randomized, phase III MARIPOSA-2 trial (NCT04988295) that
is evaluating the safety and efficacy of this regimen in the post-osimertinib setting will be reported at the ESMO Congress 2023.
HERTHENA-Lung01
In the phase I setting, the HER3-directed antibody-drug conjugate patritumab deruxtecan (HER3-DXd) has shown efficacy in patients with EGFR-mutated NSCLC harboring diverse mechanisms of resistance to EGFR TKIs [12]. The phase II HERTHENA-Lung01 trial was initiated to evaluate HER3-DXd in patients with advanced EGFR-mutated NSCLC who have progressed on prior EGFR TKI therapy and platinum-based chemotherapy. This patient group faces a substantial unmet medical need given limited benefits of salvage treatment options after chemotherapy. Moreover, CNS metastases are common in this population, and novel therapies effectuating CNS control are called for.
Patients treated in HERTHENA-Lung01 were initially randomized to either fixed-dose HER3-DXd 5.6 mg/kg or to the uptitration arm in which treatment was escalated from 3.2 mg/kg to 6.4 mg/kg. After a benefit-risk assessment supported closure of the uptitration arm, enrollment was discontinued in this group, while it continued in the fixed-dose arm. Data for 225 patients treated with HER3-DXd 5.6 mg/kg were reported by Yu et al. at WCLC 2023 [13]. The design of HERTHENA-Lung01 allowed for inactive or pretreated asymptomatic brain metastases. Fifty-one percent of patients had a history of CNS metastases, and 32 % had brain metastases at baseline. Liver metastasis was present in 33 %. The median number of previous treatment lines was 3, and 93 % had previously received third-generation EGFR TKI therapy. The primary endpoint of the HERTHENA-Lung01 trial was confirmed ORR (cORR) by BICR.
Efficacy independent of resistance mechanisms
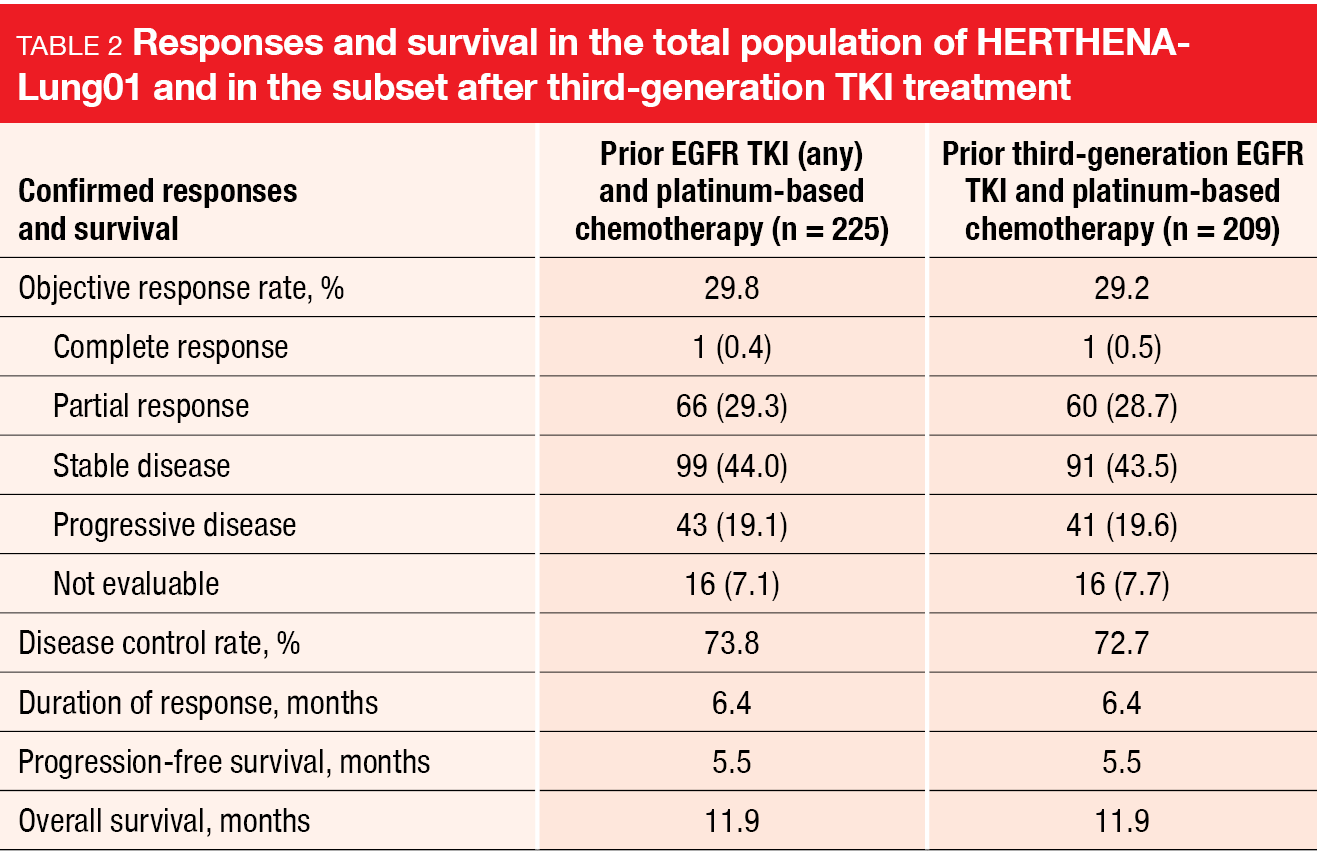
Indeed, HER3-DXd provided clinically meaningful and durable efficacy. The cORR was 29.8 % and 29.2 % in the total group and in the subset of patients after third-generation EGFR TKI treatment, respectively (Table 2). In 73.8 % and 72.7 %, respectively, disease control was obtained. Responses lasted for a median of 6.4 months in both the overall population and the subgroup pretreated with third-generation EGFR TKI therapy. Similarly, PFS was 5.5 months and OS was 11.9 months for both populations. Thirty patients had brain metastasis at baseline without prior radiotherapy. In this group, HER3-DXd gave rise to a cORR of 33.3 %, with complete remission observed in 30.0 % and a disease control rate of 76.7 %. Responses lasted for a median of 8.4 months.
Tumor reductions occurred across diverse mechanisms of EGFR TKI resistance. cORRs were 32.4 % and 27.2 % for patients with EGFR-dependent and EGFR-independent resistance mechanisms, respectively. In the group that had both of these mechanisms, the cORR was 37.5 %. When no mechanisms of EGFR TKI resistance were identified, 27.3 % of patients responded. Moreover, the treatment showed efficacy across a broad range of pretreatment tumor HER3 membrane expression levels.
The safety profile of HER3-DXd in this heavily pretreated cohort was manageable and tolerable. Nausea, thrombocytopenia, decreased appetite, neutropenia, constipation, anemia and fatigue were noted as the most common AEs, with the majority graded as 1 or 2. Hematological toxicities typically occurred early on, were transient, and were not associated with clinical sequelae. Treatment-emergent AEs (TEAEs) prompted treatment discontinuation in 7.1 % and dose reductions in 21.3 %. Adjudicated ILD occurred in 12 patients (5.3 %). Most of these (n = 8) had grade 2 ILD, although one patient died due to this complication (0.4 %). In their conclusions, the authors noted that HER3-DXd has emerged as a promising option for patients with EGFR-mutated NSCLC after failure of EGFR TKI treatment and platinum-based chemotherapy.
Trastuzumab deruxtecan in HER2-mutant disease
In the setting of metastatic HER2-mutated lung cancer, the randomized, international, non-comparative, phase II DESTINY-Lung02 trial is assessing the antibody-drug conjugate trastuzumab deruxtecan (T-DXd) at doses of 5.4 mg/kg Q3W (n = 102) and 6.4 mg/kg Q3W (n = 50). The patients enrolled in this study have already received ≥ 1 prior anticancer treatment including platinum-based chemotherapy, with a median of two lines in both arms. In the T-DXd 5.4 and 6.4 mg/kg arms, 34.3 % and 44.0 %, respectively, showed baseline CNS lesions. cORR by BICR is defined as the primary endpoint. Both patients and investigators are blinded to the dose levels.
The primary analysis of DESTINY-Lung02 reported at WCLC 2023 demonstrated deep and durable responses for both T-DXd doses [14]. cORRs were 49.0 % and 56.0 %, respectively, and disease control was observed in 93.1 % and 92.0 %, respectively. Median duration of response was 16.8 months in the 5.4 mg/kg dose group and had not been reached in the 6.4 mg/kg cohort. Responses occurred irrespective of HER2 mutation type, HER2 amplification status, and number or type of prior anticancer therapies. Median PFS was 9.9 and 15.4 months for the 5.4 mg/kg and 6.4 mg/kg doses, respectively, with 12-month PFS rates of 53 % and 45 %. Median OS was 19.5 months and had not been reached, respectively. The 12-month OS rates were 73 % and 67 %, respectively.
Both doses showed an acceptable and generally manageable safety profile, although the results favored the 5.4 mg/kg dose, which conferred a lower incidence of drug-related grade ≥ 3 TEAEs (38.6 % and 58.0 %), serious TEAEs (13.9 % and 24.0 %) as well as TEAEs associated with discontinuation (13.9 % and 20.0 %), dose reduction (16.8 % and 32.0 %), and dose interruption (26.7 % and 48.0 %). The most common grade ≥ 3 TEAEs included neutropenia (18.8 % and 36.0 %) and anemia (10.9 % and 16.0 %). Any-grade ILD was lower with the 5.4 mg/kg dose (12.9 % vs. 28.0 %). Taken together, the results of the primary analysis of DESTINY-Lung02 support the use of T-DXd 5.4 mg/kg for patients with previously treated HER2-mutated NSCLC and reinforce T-DXd as the standard of care in this population.
Phase I data for HER2 TKI therapy
The novel TKI zongertinib (BI 1810631) covalently and selectively binds to the tyrosine kinase domain (TKD) of HER2. This agent is under investigation as an oral treatment for NSCLC harboring HER2 TKD mutations, including exon 20 insertion mutations. Yamamoto et al. reported results for the ongoing phase Ia/Ia BEAMION LUNG-1 trial conducted in patients with various advanced solid tumors harboring HER2 aberrations (phase Ia) as well as patients with HER2-positive NSCLC (phase Ib, Cohort 1) [15].
In phase Ia, the maximum tolerated dose of zongertinib was not reached in 50 individuals, neither with the BID schedule nor the OD schedule. Rates of EGFR-mediated AEs were low. The zongertinib doses taken into dose optimization were 240 mg and 120 mg OD. Regarding antitumor activity in the group with NSCLC (n = 34), the analysis revealed encouraging preliminary efficacy of zongertinib. The ORR was 50 % and the disease control rate 97.1 %.
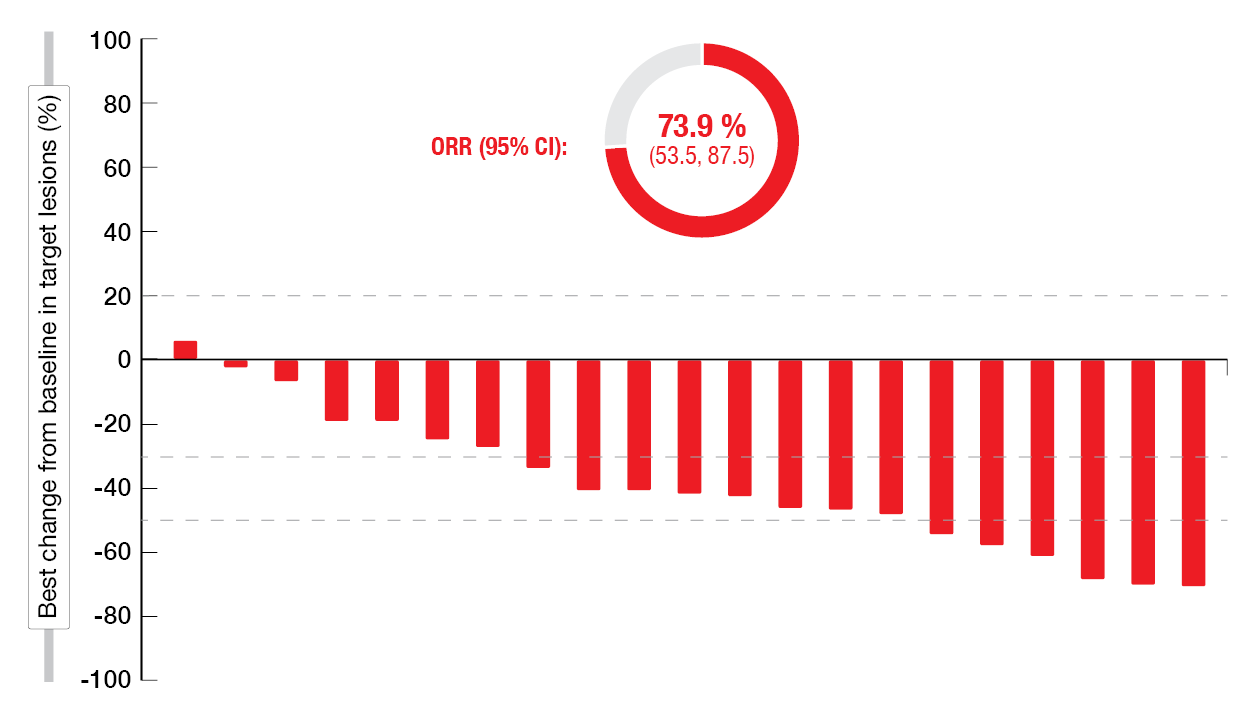
In the phase Ib setting, 42 patients have been treated to date in Cohort 1 that includes pretreated patients with HER2 TKD mutations. Dose reduction became necessary in one patient due to TRAEs (i.e., grade 3 febrile neutropenia and decreased neutrophil count). No discontinuation occurred due to AEs. At 28.6 % and 21.4 %, diarrhea and rash, respectively, represented the most frequent TRAEs of zongertinib. The initial efficacy results were promising, with an ORR of 73.9 % and a disease control rate of 91.3 % (Figure 2). The median best percentage change in target lesions from baseline was – 41.2 %.
Figure 2: Reductions in tumor volume and ORR with zongertinib in the phase Ib setting
CodeBreaK 101: sotorasib plus chemotherapy
Sotorasib has been introduced as the first-in-class KRASG12C inhibitor for the treatment of KRASG12C-mutated NSCLC. The single-arm, phase Ib CodeBreaK 101 trial is the first global study to assess the efficacy and safety of sotorasib in addition to carboplatin/pemetrexed in patients with KRASG12C-mutated advanced NSCLC. Sotorasib 960 mg OD orally is administered together with pemetrexed and carboplatin Q3W for four cycles during the induction phase. During the maintenance phase, the treatment consists of sotorasib plus pemetrexed Q3W. While 25 individuals are being treated in the first-line setting, 13 patients constitute the second-line cohort.
According to the results presented by Clarke et al. at WCLC 2023, sotorasib plus pemetrexed/carboplatin showed predictable toxicity and promising clinical activity [16]. Common TRAEs were consistent with sotorasib and platinum-doublet-based regimens. Neutropenia/neutrophil count decreases occurred most commonly (all grades, 53 %; grade 3-4, 32 %), followed by anemia (all grades, 39 %; grade 3-4; 21 %) and thrombocytopenia/platelet count decrease (all grades, 37 %; grade 3-4; 16 %). TRAEs led to treatment discontinuation in 12 % and 31 % in the first-line and second-line groups, respectively. No fatal AEs occurred.
With regard to efficacy, the analysis yielded ORRs of 65 % and 54 % in the first- and second-line settings, respectively. Disease control resulted in 100 % and 85 %, respectively. The ORRs were similar across PD-L1 expression levels, ranging from 50 % to 75 %. After a median follow-up of 3.0 months, the observations indicated rapid and durable responses. PFS and OS findings were not mature yet. A longer follow-up is ongoing to assess the durability of the combination. Furthermore, first-line sotorasib plus pemetrexed/carboplatin is being tested against pembrolizumab plus pemetrexed/carboplatin in patients with KRASG12C-mutated, PD-L1–negative NSCLC in the phase III CodeBreaK 202 trial (NCT05920356).
Local consolidation in addition to brigatinib
ALK TKIs are the standard of care for the treatment of ALK-rearranged metastatic NSCLC, although it has been shown that approximately 95 % of patients with initial remission exhibit incomplete responses that result in residual disease, which might elicit acquired resistance [17]. The BRIGHTSTAR study tested the hypothesis that minimizing or eliminating residual disease with local consolidation therapy (LCT) might delay the development of resistance in all-comer patients with ALK-positive, stage IV or recurrent NSCLC. These were either TKI-naïve or had received brigatinib for ≤ 8 weeks, and had ≥ 1 site of residual disease. Patients without disease progression according to CT/PET and brain MRI after 8 weeks of brigatinib therapy received brigatinib until progression in addition to local LCT with radiotherapy, surgery, or both. In patients with < 3 active sites of disease, LCT was administered to all sites, while in those with > 3, the physicians decided which sites to treat. The primary objective of BRIGHTSTAR was the safety and tolerability of the combined approach.
Results for 34 patients were presented at WCLC 2023 by Elamin et al. [18]. In this group, 82 % had > 3 metastases at baseline. At 8 weeks, 79 % and 21 % had achieved partial response and stable disease with brigatinib treatment, respectively. Thirty-two patients successfully completed LCT as planned. Radiation was administered in 79 %, surgery in 9 %, and both in 6 %. In the remaining cases, residual disease was not amenable to LCT after induction therapy, or consent was withdrawn. Complete LCT to all sites of residual disease was possible in 62 %. In 10 of 29 patients who underwent radiotherapy, brigatinib therapy was continued during treatment. Surgical procedures included lobectomy (n = 3), sub-lobar resection (n = 1) and adrenalectomy (n = 1).
Brigatinib plus LCT was demonstrated to be safe. Six grade ≥ 3 LCT-related AEs occurred, which included grade 4 bronchopulmonary hemorrhage, grade 3 anemia, grade 3 pneumonitis, grade 3 esophagitis, and grade 3 vomiting and nausea. No grade 5 events related to LCT were noted. The combined approach yielded promising results compared to historical outcomes. The PFS rates at 2 and 3 years were 80 % and 66 %, respectively. In the pivotal ALTA-1L trial that had assessed first-line, single-agent brigatinib, these rates had been 56 % and 47 %, respectively, in patients who had not progressed at 12 weeks [19].
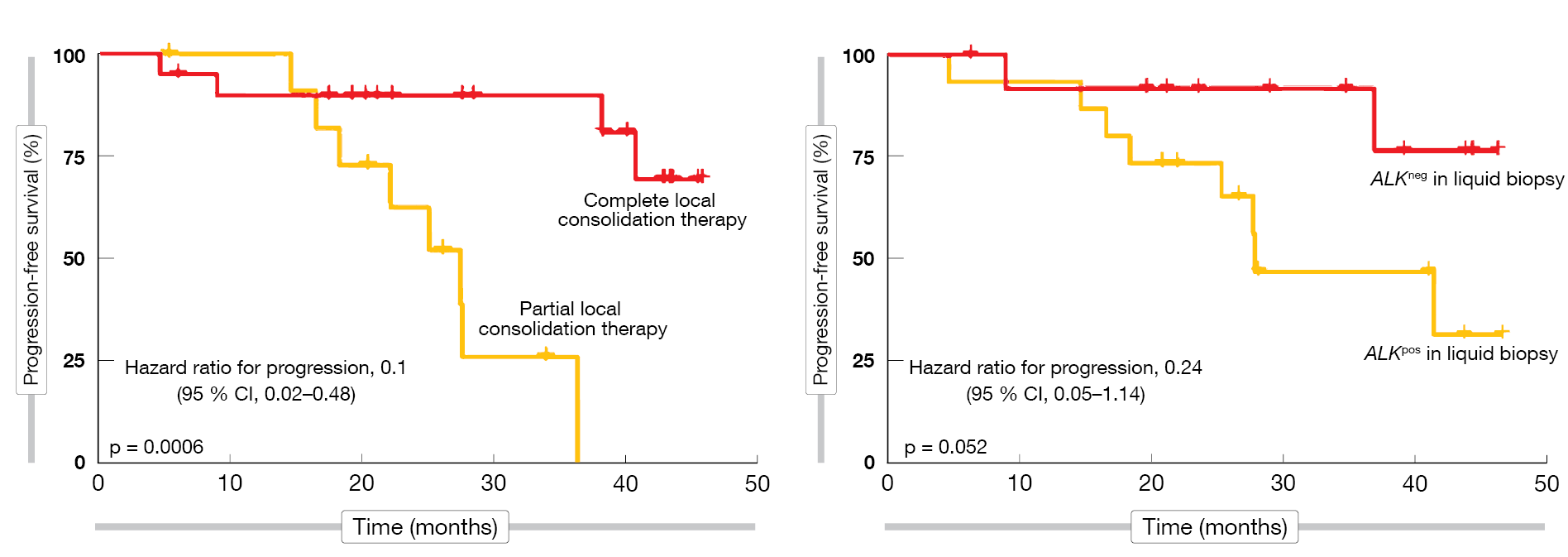
In addition, the authors explored predictors of outcome. Improved PFS with LCT was seen for complete vs. partial LCT and for ALK-negative status in the plasma at baseline according to liquid biopsy (Figure 3). Also, lower tumor volume at baseline as well as after induction therapy predicted increased treatment benefit. On the other hand, the number of metastases at baseline (oligometastatic vs. polymetastatic disease) did not affect the outcomes. The randomized BRIGHTSTAR-2 trial will compare LCT and chemotherapy as intensification strategies with brigatinib monotherapy in the first-line setting of ALK-positive NSCLC.
Figure 3: Predictors of progression-free survival benefit with brigatinib plus local consolidation therapy (LCT): complete LCT (left) and ALK negativity at baseline
REFERENCES
- Hosomi Y et al., Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 Study. J Clin Oncol 2020; 38(2): 115-123
- Noronha V et al., Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol 2019; 38(2): 124-136
- Oizumi S et al., Updated survival outcomes of NEJ005/TCOG0902: a randomised phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations. ESMO Open 2018; 3(2): e000313
- Saito R et al., Phase 2 study of osimertinib in combination with platinum and pemetrexed in patients with previously untreated EGFR-mutated advanced non-squamous non-small cell lung cancer: The OPAL Study. Eur J Cancer 2023; 185: 83-93
- Jänne PA et al., Osimertinib with/without platinum-based chemotherapy as first-line treatment in patients with EGFRm advanced NSCLC (FLAURA2). WCLC 2023, abstract PL03.13
- Choudhury NJ et al., Molecular biomarkers of disease outcomes and mechanisms of acquired resistance to first-line osimertinib in advanced EGFR-mutant lung cancers. J Thorac Oncol 2023; 18(4): 463-475
- Han B et al., Efficacy of pemetrexed-based regimens in advanced non-small cell lung cancer patients with activating epidermal growth factor receptor mutations after tyrosine kinase inhibitor failure: a systematic review. Onco Targets Ther 2018; 11: 2121-2129
- Rios-Hoyo A et al., Acquired mechanisms of resistance to osimertinib-the next challenge. Cancers (Basel) 2022; 14(8): 1931
- Wang Y et al., Clinical analysis by next-generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer 2018; 118: 105-110
- Kim TM et al., Tepotinib + osimertinib in EGFR-mutant NSCLC with MET amplification following 1L osimertinib: INSIGHT 2 primary analysis. WCLC 2023, abstract OA21.05
- Lee SH et al., Amivantamab, lazertinib plus platinum-based chemotherapy in post-TKI EGFR-mutated advanced NSCLC: updated results from CHRYSALIS-2. WCLC 2023, abstract MA13.06
- Jänne PA et al., Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov 2022; 12(1): 74-89
- Yu HA et al., Patritumab deruxtecan (HER3-DXd) in EGFR-mutated NSCLC following EGFR TKI and platinum-based chemotherapy: HERTHENA-Lung01. WCLC 2023, abstract OA05.03
- Jänne PA et al., Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small cell lung cancer: primary results of DESTINY-Lung02. WCLC 2023, abstract MA13.10
- Yamamoto N et al., BEAMION LUNG-1, a phase IA/IB trial of the HER2 TKI zongertinib (BI 1810631) in patients with advanced solid tumors with HER2 aberrations. WCLC 2023, abstract MA13.08
- Clarke JM et al., CodeBreaK 101: Safety and efficacy of sotorasib with carboplatin and pemetrexed in KRASG12C-mutated advanced NSCLC. WCLC 2023, abstract MA06.05
- Biovona TG, Doebele RC, A framework for understanding and targeting residual disease in oncogene-driven solid cancers. Nat Med 2016; 22(5): 472-478
- Elamin YY et al., Local consolidative therapy with brigatinib in tyrosine kinase inhibitor-naïve ALK-rearranged metastatic NSCLC. WCLC 2023, abstract OA22.04
- Camidge DR et al., Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: Final results of phase 3 ALTA-1L trial. J Thorac Oncol 2021; 16(12): 2091-2108
© 2023 Springer-Verlag GmbH, Impressum