Exploring established and novel EGFR-directed agents
PROs & dose modifications in LUX-Lung 7
The phase IIb LUX-Lung 7 trial was a head-to-head comparison of the second- generation ErbB family blocker afatinib and the first-generation reversible EGFR TKI gefitinib in patients with treatment-naïve, EGFR-mutation-positive, advanced (stage IIIB/IV) adenocarcinoma of the lung. According to the primary analysis, patients treated with afatinib derived significant PFS, ORR and time-to-treatment-failure benefits compared to those who received gefitinib [1]. The OS data are currently immature.
Patient-reported outcomes (PROs) as well as post-hoc analyses of the impact of afatinib dose adjustments on PFS, management of AEs, and PROs were presented at the ASCO Congress by Hirsh et al. [2]. Afatinib dose escalation or reduction was permitted according to a pre-specified dose adjustment scheme. The incidence and severity of common AEs before and after dose reductions from 40 mg were assessed. Also, the investigators compared PROs and PFS between patients who had dose reductions within 6 months and those who received at least 40 mg for the first 6 months.
Preserved efficacy with dose reductions
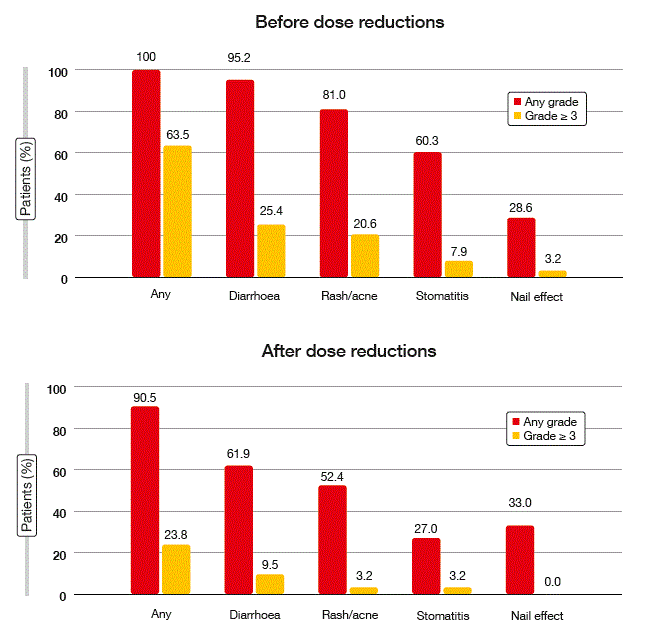
Dose reductions occurred more often with afatinib than with gefitinib. Gefitinib is available in only one dose strength, as opposed to afatinib (20 mg, 30 mg, 40 mg, 50 mg). Thirty-nine percent of patients treated with afatinib 40 mg had dose reductions to 30 mg; 13 % had further dose reductions to 20 mg. However, the rates of drug-related discontinuations due to AEs were similar across arms, which suggested that dose reductions effectively managed AEs. Indeed, dose adjustments led to decreases in the incidence and severity of drug-related AEs (Figure).
Figure: Reduction in incidence and severity of treatment-related AEs through dose modifications of afatinib
With regard to PROs, according to the EQ-5D™ health status self-assessment questionnaire, similar improvements were observed in both study groups. There were no significant or clinically meaningful differences between the afatinib and gefitinib treatment arms with respect to mean EQ-5D or EQ-VAS scores. Reductions in the afatinib doses did not diminish the treatment effects on PROs. Also, PFS did not differ between patients receiving doses of < 40 mg or ≥ 40 mg during the first 6 months of treatment. Median PFS was 12.8 and 11.0 months in patients with and without dose reductions, respectively. In comparison, median PFS for the gefitinib arm was 10.9 months, according to the primary analysis [1].
Overall, the afitinib dose adjustments enabled patients to remain on treatment. As observed in the LUX-Lung 3 and LUX-Lung 6 trials [3], tolerability-guided reductions in afatinib doses constituted an effective measure to reduce treatment-related AEs without affecting therapeutic efficacy.
VeriStrat analysis of the LUX-Lung 8 trial
Afatinib was compared with erlotinib in the open-label, phase III LUX-Lung 8 trial that enrolled 795 patients with advanced squamous-cell carcinoma of the lung, who had progressed after at least four cycles of platinum-based chemotherapy. Patients treated with afatinib showed significant benefits for OS, PFS and disease control [4]. Goss et al. presented data obtained with VeriStrat [5], a serum-protein mass spectrometry test that has demonstrated prognostic and predictive utility for EGFR-targeted therapies in NSCLC [6]. The investigators assessed the predictive ability of VeriStrat in LUX-Lung 8, using OS as the primary efficacy variable. To that end, serum pre-treatment samples from 675 patients were classified as ‘good’ (VS-G) or ‘poor’ (VS-P) based on pre-defined reference groups. Clinical outcomes were analysed with respect to the VeriStrat status in the overall population and in pre-defined subgroups.
In the VS-G group (n = 412), median OS was 11.5 and 8.9 months for afatinib and erlotinib, respectively (HR, 0.79; p = 0.03); median PFS was 3.3 and 2.0 months, respectively (HR, 0.73; p = 0.005). For patients classified as VS-P (n = 263), median OS was 4.7 and 4.8 months, respectively (HR, 0.90; not significant), and median PFS was 1.9 months for both afatinib and erlotinib (HR, 0.96; not significant). In patients treated with afatinib, both OS and PFS were longer in the VS-G group than in the VS-P group (OS: HR, 0.40; p < 0.0001; PFS: HR, 0.56; p < 0.0001). According to multivariate analysis, VeriStrat is an independent predictor of OS and PFS in afatinib-treated patients regardless of ECOG performance status, best response to first-line therapy, age, and ethnicity. However, no interactions were demonstrated between VeriStrat classification and treatment group for OS or PFS.
Overall, VeriStrat conferred a strong independent stratification effect in patients with relapsed/ refractory squamous- cell carcinoma of the lung treated with afatinib in the LUX-Lung 8 trial. In these difficult-to-treat patients, afatinib therapy gave rise to significantly superior OS and PFS, as compared to erlotinib in the VS-G group.
Updated data on rociletinib: TIGER-X
TKIs that inhibit mutant forms of the EGFR gene have two important limitations: the inhibition of wild-type EGFR leads to cutaneous toxicity and diarrhoea, and the efficacy of treatment is limited by the emergence of the EGFR T790M acquired resistance mutation in approximately 60 % of patients. Rociletinib was therefore designed as an oral, irreversible inhibitor of the activating mutation exon 19 and the L858R point mutation in exon 21, as well as of the acquired resistance mutation T790M. It has only minimal activity against wild-type EGFR.
Goldman et al. reported updated results from the phase I/II TIGER-X study that investigated rociletinib in patients with advanced or recurrent, centrally confirmed T790M-positive NSCLC [7]. After the dose-expansion phase I part of the trial, patients who had progressed on one or two EGFR TKIs entered the expansion cohort (phase II). Rociletinib was tested at three doses (500 mg BID, 625 mg BID, 750 mg BID) in 548 patients. N-acetyl transferase 2 (NAT2) genotype polymorphism was assessed for a subgroup in all three dosing cohorts.
According to the investigators, rociletinib therapy led to a confirmed ORR of 33.9 %. This is lower than the response rates reported previously [8, 9]. In the three dosing groups, responses proved durable, at a median of 8.9, 9.0, and 7.1 months, respectively. PFS was 5.7, 5.0, and 4.3 months, respectively.
The most common AEs of any grade across all doses included hyperglycaemia, diarrhoea, nausea, fatigue, and decreased appetite. Hyperglycaemia, QTc prolongation, and fatigue counted among the most frequently reported grade-3/4 AEs. Cataracts were found to be common in patients receiving rociletinib for prolonged periods of time, which is why visual symptoms should be investigated promptly. Based on the NAT2 genotype results, patients were classified as having a slow (n = 196), intermediate (n = 148), or rapid (n = 38) acetylator phenotype. Slow acetylators showed a tendency to develop hyperglycaemia, QTc prolongation, or other cardiac disorders. The clinical development of rocilitinib has recently been stopped by Clovis Inc.
Innovative 3rd-generation EGFR-mutant-specific TKI: olmutinib
Olmutinib is an oral, third-generation TKI with EGFR-mutant-specific activity against deletion 19, L858R, and T790M. It does not inhibit wild-type EGFR. The safety, tolerability, pharmacokinetics and preliminary activity of olmutinib were evaluated in an open-label, multicentre phase I/II trial in Korean patients with EGFR-TKI-pretreated NSCLC. Seventy-six patients with T790M mutation received olmutinib 800 mg OD in the phase II part of the study. These patients had experienced progression on at least one prior EGFR TKI.
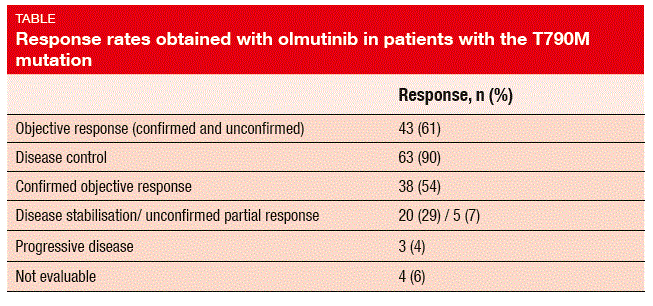
Sixty-one percent of patients achieved tumour shrinkage that qualified for objective response (Table). In 84 %, onset of tumour response occurred by week 6. Disease control was seen for 90 %. The median duration of response was 8.3 months. Patients with one prior systemic treatment obtained a median PFS of 8.8 months, while in those with two or more prior regimens, PFS was 6.8 months. With regard to tolerability, patients most commonly reported diarrhoea, pruritus, rash, and nausea, which were mainly of mild-to-moderate in intensity. Four patients discontinued treatment due to AEs (upper abdominal pain and vomiting, interstitial lung disease, peripheral neuropathy, skin exfoliation). QT prolongation and hyperglycaemia were not observed.
The authors concluded that olmutinib showed meaningful clinical activity with a favourable safety profile at the recommended phase II dose of 800 mg OD. An ongoing global phase II trial, ELUXA 1, is further assessing the efficacy and safety of olmutinib in patients with T790M-positive NSCLC.
REFERENCES
- Park K et al., Afatinib versus gefitinib as firstline treatment of patients with EGFR mutationpositive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17(5): 577-589
- Hirsh V et al., First-line afatinib versus gefitinib for patients with EGFR mutation-positive NSCLC (LUX-Lung7): patient-reported outcomes and impact of dose modifications on efficacy and adverse events. J Clin Oncol 34, 2016 (suppl; abstr 9046)
- Schuler M et al., First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016; 11(3): 380-390
- Soria JC et al., Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16(8): 897-907
- Goss GD et al., Evaluation of VeriStrat, a serum proteomic test, in the randomized, open-label, phase 3 LUX-Lung 8 (LL8) trial of afatinib (A) versus erlotinib (E) for the second-line treatment of advanced squamous cell carcinoma (SCC) of the lung. J Clin Oncol 34, 2016 (suppl; abstr e20510)
- Gregorc V, et al., Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol 2014; 15(7): 713-721
- Goldman JW et al., Updated results from TIGER- X, a phase 1/2 open-label study of rociletinib in patients with advanced, recurrent T790M-positive non-small cell lung cancer. J Clin Oncol 34, 2016 (suppl; abstr 9045)
- Sequist LV et al., Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015; 372: 1700-1709
- Sequist LV et al., Efficacy of rociletinib (CO- 1686) in plasma-genotyped T790M-positive non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol 33, 2015 (suppl; abstr 8001)
- Park K et al., Olmutinib (BI 1482694; HM61713), an EGFR mutant-specific inhibitor, in T790M+ NSCLC: efficacy and safety at the RP2D. J Clin Oncol 34, 2016 (suppl; abstr 9055)
More posts
PFS improvement due to local therapy in oligometastatic NSCLC
PFS improvement due to local therapy in oligometastatic NSCLC Evidence suggests
Locally advanced NSCLC: oral vinorelbine shows better safety profile than etoposide
Locally advanced NSCLC: oral vinorelbine shows better safety profile than etoposide
ULTIMATE: chemotherapy plus bevacizumab beyond first line
ULTIMATE: chemotherapy plus bevacizumab beyond first line As chemotherapy in th
New approaches are raising hope for SCLC patients
New approaches are raising hope for SCLC patients Only minor progress has been
Mutational analysis: on the road to refined standards
Mutational analysis: on the road to refined standards LCMC II The Lung Cancer M
“The importance of first-line and second-line targeted agents is obvious”
“The importance of first-line and second-line targeted agents is obvious” Nir P