Immunotherapy: updates on clinical trials and other insights
Nivolumab plus ipilimumab: CheckMate 012
Besides targeted drugs for driver mutations, immunotherapies represent one of the two recent major advancements of the past decade for the treatment of metastatic non–small-cell lung cancer (NSCLC). Nivolumab and ipilimumab enhance T-cell antitumour activity through distinct and complementary mechanisms. The combination of these two agents has already been approved in the US and EU for metastatic melanoma. In NSCLC, nivolumab monotherapy is approved as a second-line treatment for locally advanced or metastatic disease, while first-line standard treatment still consists of platinum-doublet chemotherapy. Progress towards improved first-line treatment options has plateaued over the last decade, and the need for improvement in this clinical setting is critical.
Therefore, the 3-arm, randomised, phase I, CheckMate 012 trial examined the role of combination immunotherapy in patients with advanced NSCLC (stage IIIB/IV) of any histology. Treatment consisted of nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks, or nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks. Previous data had indicated that efficacy was greatest in the two arms that received nivolumab 3 mg/ kg. The updated analysis presented at the ASCO Congress was performed after an extended follow-up in these two groups, which comprised 38 patients (nivolumab plus ipilimumab every 12 weeks) and 39 patients (nivolumab plus ipilimumab every 6 weeks), respectively [1]. Safety and tolerability were defined as the primary endpoints.
Improved tolerability and promising efficacy
As compared to older combination regimens included in the CheckMate 012 trial that used higher or more frequent doses of ipilimumab, these dosing schedules showed improved tolerability and a manageable safety profile. Treatment-related grade 3/4 adverse events (AEs) occurred in 37 % and 33 %, respectively, and led to discontinuation at a third of the rate seen with the older study arms (5 % and 8 %, respectively). No treatment-related deaths were observed. Reassuringly, the overall incidence of grade 3/4 immune-related AEs was low across all treatment arms. These data were similar to a separate arm of the CheckMate 012 trial that used nivolumab monotherapy 3 mg/kg every 2 weeks.
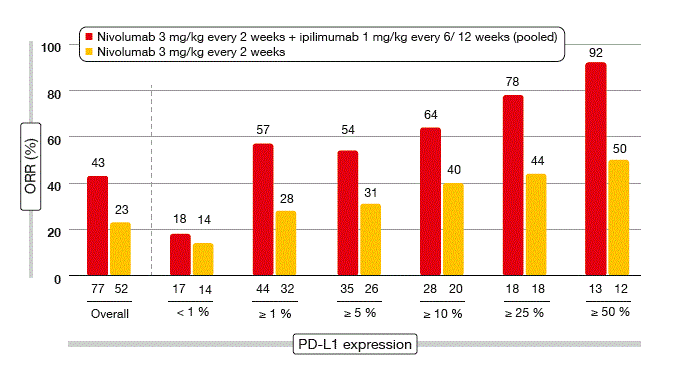
The analysis yielded promising efficacy, with overall response rates (ORRs) of 47 % and 39 %, respectively. These ORRs exceed those obtained with nivolumab monotherapy (23 %). The median duration of response was not yet reached. According to a pooled biomarker analysis, efficacy was enhanced with increasing PD-L1 expression, and patients treated with the combination fared better than the historical nivolumab-only group across all PD-L1 expression levels (Figure 1). Also, superiority of the combination over nivolumab monotherapy was observed in never smokers and current/ former smokers alike. Patients with EGFR mutations showed markedly higher ORRs with nivolumab plus ipilimumab than with nivolumab alone. Responses obtained with the combination tended to be both deep and durable, and were achieved early in most cases. The schedule containing nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks is being evaluated in further studies, including the phase III CheckMate 227 trial.
Figure 1: CheckMate 012: pooled ORR analysis of two schedules of nivolumab plus ipilimumab across different PD-L1 expression levels, as compared to nivolumab monotherapy
Durable survival benefit of nivolumab in CheckMate 017 and 057
Nivolumab monotherapy demonstrated a significant overall survival (OS) benefit compared with docetaxel in advanced NSCLC in the phase III Check- Mate 017 (squamous histology) [2] and CheckMate 057 (non-squamous histology) trials [3]. Borghaei et al. presented the updated OS and safety results from these two studies, based on a follow-up of ≥ 2 years [4]. Also, exploratory analyses of the association between baseline serum cytokine profiles and OS were conducted for both histologies.
In both trials, nivolumab demonstrated durable, long-term OS and progression- free survival (PFS) compared with docetaxel. The differences in OS and PFS rates between the nivolumab and docetaxel arms remained consistent from 1 to 2 years. At two years, 23 % vs. 8 % of patients in the nivolumab and docetaxel arms, respectively, were alive in the CheckMate 017 trial (HR, 0.62). For CheckMate 057, these percentages were 29 % vs. 16 % (HR, 0.75). In Check- Mate 057, as in the primary analysis, PD-L1 expression level was associated with magnitude of OS benefit. Treatment-related AEs were reported in fewer nivolumab-treated patients than in docetaxel-treated patients in both studies. Overall, AE rates at 2 years resembled those at 1 year.
The cytoscores, which reflect the cytokine profile at baseline, appeared to be associated with prognosis in both squamous and non-squamous disease, but these results are only hypothesisgenerating and require prospective validation. Cytoscores were not associated with treatment effects of nivolumab over docetaxel.
Long-term results for pembrolizumab
Based on the findings obtained in the large multi-cohort phase Ib KEYNOTE-001 study [5, 6], pembrolizumab received accelerated approval in the United States for the treatment of advanced NSCLC that expresses PD-L1 and has progressed after platinum-containing chemotherapy and (in the case of EGFR or ALK positivity) an approved EGFR or ALK inhibitor. KEYNOTE-001 demonstrated a correlation between higher PD-L1 expression and improved outcomes.
The long-term analysis of the KEYNOTE-001 trial showed that pembrolizumab monotherapy provides sustained OS benefit in patients with advanced NSCLC [7]. Increased PD-L1 expression was associated with increased survival benefit. Pembrolizumab continued to have a manageable safety profile; no unexpected toxicities occurred during the long-term followup. Along with the KEYNOTE-010 trial, which demonstrated OS improvement with pembrolizumab in patients with previously treated NSCLC and PD-L1 expression of ≥1 % on tumour cells [8], these data support PD-L1 as a predictive biomarker for pembrolizumab, and confirm the manageable safety profile of this agent.
OS improvement with atezolizumab becomes apparent over time
The engineered and humanised anti-PD-L1 antibody atezolizumab was compared to docetaxel in the multi-centre, randomised, open-label, phase II, POPLAR study in patients with previously treated locally advanced or metastatic NSCLC who progressed during or after platinum-based therapy. At a minimum follow-up of 13 months, the primary analysis was conducted, which revealed an OS benefit of atezolizumab over docetaxel in both unselected and PD-L1-selected patients [9, 10]. Increasing PD-L1 expression on tumour cells and/ or immune cells was associated with increasing OS benefit. The survival curves showed late separation, underscoring the need for long-term follow-up to fully capture the benefit of this anti-PD-L1 therapy.
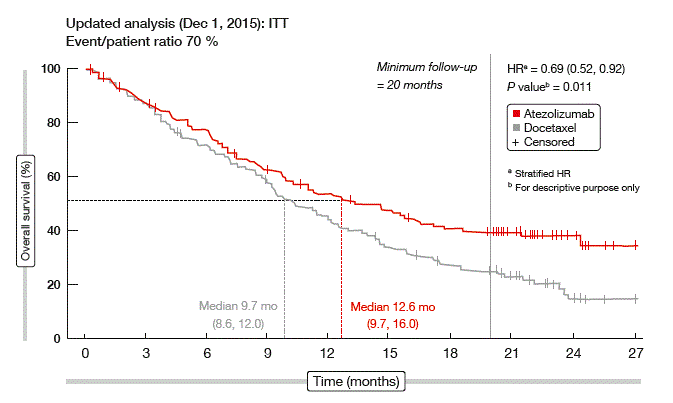
Therefore, Smith et al. presented an updated analysis after a minimum follow-up of 20 months [11]. This showed further separation of the survival curves in the ITT population (Figure 2). Consistent with the previous pattern, OS hazard ratios (HRs) improved in favour of atezolizumab over time. The OS benefit was observed in all PD-L1 subgroups. Also, survival curves for histology subgroups showed continued separation over time, with the improvement in HRs more pronounced in the squamous NSCLC subgroup. PFS and ORR were similar across the atezolizumab and docetaxel arms in the ITT population; here, the data did not change significantly from the primary analysis.
Figure 2: Updated OS findings with atezolizumab vs. docetaxel in the POPLAR trial
As the authors noted, the lack of correlation between the OS benefit and the PFS and ORR findings implies that OS improvement with atezolizumab might extend beyond disease progression by RECIST. Responses observed with atezolizumab were durable, however (median, 18.6 vs. 7.2 months with atezolizumab and docetaxel, respectively). Together, these results provide further evidence that survival benefits with atezolizumab extend to all patients with NSCLC.
Durvalumab shows efficacy in squamous and non-squamous disease
A multicentre, open-label, dose-escalation and dose-expansion, phase I/II trial investigated the safety and clinical efficacy of durvalumab in patients with advanced, treatment-naïve NSCLC [12]. Durvalumab is a selective, high-affinity, engineered human monoclonal anti-PD-L1 antibody. PD-L1 expression was prospectively evaluated (high: ≥ 25 % tumour cell staining; low or negative: < 25 % tumour cell staining). Fifty-nine patients who had not received prior systemic therapy for advanced disease were treated. Forty-nine of these showed high PD-L1 expression.
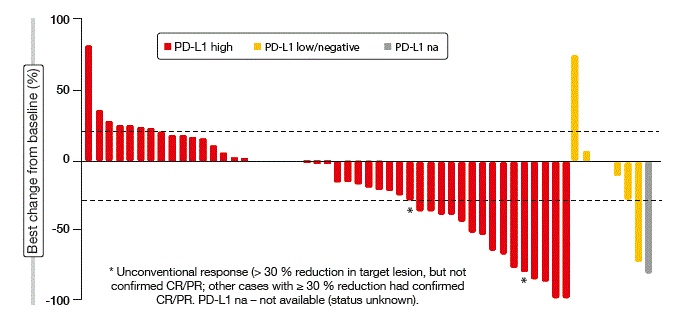
Durvalumab 10 mg/kg every 2 weeks was demonstrated to have a manageable safety profile. ORR was 27 %; in patients with high PD-L1 expression, re-sponses occurred in 29 %, and in those with low or negative expression, in 11 %. One patient with high expression experienced complete remission. Reductions of target lesions were observed in patients with both high and low/ negative PD-L1 expression and in one patient with an unknown PD-L1 expression status (Figure 3). ORR was similar regardless of histology (squamous vs. non-squamous) for patients with high PD-L1 expression. In three patients who had non-squamous disease and low or negative PD-L1 expression, no responses occurred. Current or former smokers with high expression showed an ORR of > 30 %. Responses were generally rapid and durable; at data cut-off , they were ongoing in 69 %. Durvalumab is currently being investigated across a range of studies in treatment-naïve patients with advanced NSCLC.
Figure 3: Best changes from baseline in tumour size by PD-L1 expression obtained with durvalumab
JAVELIN: avelumab in chemotherapy-refractory mesothelioma
Approximately 3,000 cases of malignant mesothelioma are diagnosed each year in the US. There are no FDA-approved treatment options for patients who progress after fi rst-line chemotherapy. The international, multi-cohort, dose-escalation and dose-expansion, phase Ib, JAVELIN trial investigated the fully human anti-PD-L1 antibody avelumab in mesothelioma on the basis that PD-L1 is expressed on the surface of mesothelioma cells [13]. JAVELIN enrolled a total of 1,600 patients with different tumour types. Fifty-three patients with advanced, unresectable, pleural or peritoneal mesothelioma, who had progressed after treatment with platinum and pemetrexed, received avelumab 10 mg/kg every 2 weeks. PD-L1 expression status was assessed, revealing the presence of any staining intensity (≥ 1 % of tumour cells) in 51.3 % of cases.
Avelumab monotherapy led to a disease control rate of 56.6 %, which was mainly due to disease stabilisation (47.2 %). Five patients developed partial responses (9.4 %), which were ongoing in four of them at last follow-up. The median duration of response was not reached. ORR and PFS did not differ according to PD-L1 expression levels.
Overall, median PFS was 17.1 weeks. Avelumab showed an acceptable safety profile. Most treatment-related AEs were grades 1 or 2. Immune-mediated AEs of any grade were seen in 13.2 %, but grade 3 events occurred in only 1.9 %. Ongoing follow-up will further characterise the durability of the clinical activity.
Is treatment beyond RECIST progression feasible?
In the context of immunotherapy, there is uncertainty around tumour reductions according to RECIST as an endpoint, because the assessment of shrinkage appears to underestimate the true magnitude of benefit in terms of survival. Th is might be justified, as conventional response criteria are based on traditional cytotoxic chemotherapy, and tumour flare or pseudo-progression can lead to early treatment discontinuation. Anecdotal cases of decreases in tumour size after initial RECIST-defined progression have led to trials allowing for treatment past RECIST-defined first progression.
A retrospective exploratory analysis presented at the ASCO Congress described findings in patients with metastatic NSCLC who were treated with anti-PD-1 therapy past conventional progression (treatment past progression, TPP) [14]. The investigators pooled three multi-centre clinical trials that had been submitted to the FDA, which evaluated anti-PD-1 monotherapy in 535 patients who progressed after initial therapy. From these, 121 patients receiving TPP were identified. Changes in tumour burden from radiographic tumour measurement data following RECISTdefined progression were evaluated.
Compared to all anti-PD-1-treated patients (n = 535), the subgroup with TPP showed a slightly higher frequency of non-squamous histology (59 % vs. 54 %). Most patients had only had one prior line of chemotherapy, and all of them had an ECOG performance status of 0 or 1, although ECOG 0 was more frequent in the TPP group (36 % vs. 25 %). Patients who received TPP initially progressed per RECIST due to unequivocal progression of non-target lesions (38 %), appearance of new lesions (32 %), or increases of ≥ 20 % from nadir in target lesions (30 %). Overlaps of 2 causes or all 3 causes were observed.
A small, but not negligible effect
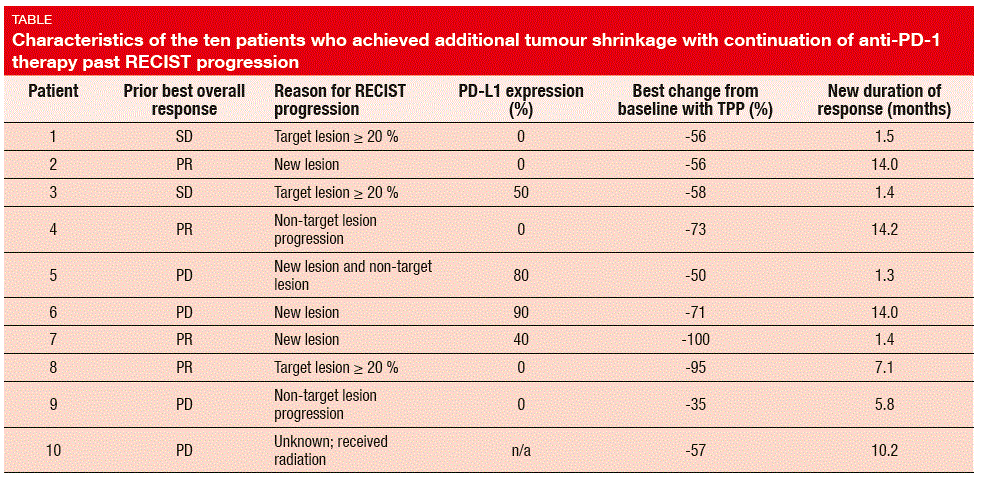
Of the 121 patients who received TPP, 10 (8.3 %) experienced additional tumour shrinkage, which was defined as a subsequent decrease in target lesions of ≥ 30 %, as compared to baseline. The Table summarises the characteristics of these patients. This group represents 1.9 % of all patients treated with anti- PD-1 agents in these trials. The best tumour reductions compared to baseline during TPP ranged from 35 % to 100 % (median, 58 %). With TPP, the median duration of responses after a ≥ 30 % reduction was not reached. At the time of data capture, at least 5 of 10 patients had responses of at least 6 months, and 3 patients had ongoing responses of over 1 year.
Moreover, the best reductions in target lesions were in patients who attained at least 30 % reduction, compared to the overall nadir measurements. Seven of the 10 patients met these criteria. The best tumour shrinkage compared to nadir ranged from 13 % to 100 %, with a median reduction of 35 %. Durations of new responses showed a wide range, from 1.3 months to > 1 year.
According to the authors’ conclusions, it is unclear whether the observed tumour reductions were due to TTP or to a delayed effect of the immunotherapy the patients had received previously. This implies that the risk of continued treatment (immune-related adverse reactions) after first progression should be balanced against the possibility of further tumour shrinkage. As more knowledge is gained with the use of immunotherapies, it might be possible to better identify patients who are more likely to benefit from TPP (e. g., biomarkers, patient characteristics). Randomised controlled trials are needed to prospectively establish any benefit of treating patients past first progression. However, TPP is unlikely to significantly change major FDA regulatory endpoint results or benefit/ risk determination.
REFERENCES
- Hellmann MD et al., CheckMate 012: safety and efficacy of first-line nivolumab and ipilimumab in advanced NSCLC. J Clin Oncol 34,
2016 (suppl; abstr 3001) - Brahmer J et al., Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015; 373: 123-135
- Borghaei H et al., Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015; 373:
1627-1639 - Borghaei H et al., Nivolumab vs. docetaxel in patients with advanced NSCLC: CheckMate 017/057 2-year update and exploratory cytokine
profile analyses. J Clin Oncol 34, 2016 (suppl; abstr 9025) - Garon EB et al., Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018-2028
- Chatterjee M et al., Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol. REFERENCES 2016 Apr 26. pii: mdw174. [Epub ahead of print]
- Hui R et al., Long-term overall survival for patients with advanced NSCLC enrolled in the KEYNOTE-001 study of pembrolizumab. J Clin Oncol 34, 2016 (suppl; abstr 9026)
- Herbst RS et al., Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540-1550
- Vansteenkiste J et al., Atezolizumab monotherapy vs docetaxel in 2L/3L non-small cell lung cancer: Primary analyses for efficacy, safety and predictive biomarkers from a randomized phase II study (POPLAR). ECC 2015, abstract 14LBA, Eur J Cancer 2015; 51 (Suppl 3): 716-717
- Fehrenbacher L et al., Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387(10030): 1837-1846
- Smith DA et al., Updated survival and biomarker analyses of a randomized phase II study of atezolizumab vs. docetaxel in previously treated NSCLC (POPLAR). J Clin Oncol 34, 2016 (suppl; abstr 9028)
- Antonia S et al., Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-L1 antibody, in treatment-naïve patients with advanced non-small cell lung cancer. J Clin Oncol 34, 2016 (suppl; abstr 9029)
- Hassan R et al., Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol 34, 2016 (suppl; abstr 8503)
- Kazandjian D et al., Characterization of patients treated with a programmed cell death protein 1 inhibitor (anti-PD-1) past RECIST progression from a pooled analysis of metastatic non-small cell lung cancer (mNSCLC) trials. J Clin Oncol 34, 2016 (suppl; abstr 3000)
More posts
PFS improvement due to local therapy in oligometastatic NSCLC
PFS improvement due to local therapy in oligometastatic NSCLC Evidence suggests
Locally advanced NSCLC: oral vinorelbine shows better safety profile than etoposide
Locally advanced NSCLC: oral vinorelbine shows better safety profile than etoposide
ULTIMATE: chemotherapy plus bevacizumab beyond first line
ULTIMATE: chemotherapy plus bevacizumab beyond first line As chemotherapy in th
New approaches are raising hope for SCLC patients
New approaches are raising hope for SCLC patients Only minor progress has been
Mutational analysis: on the road to refined standards
Mutational analysis: on the road to refined standards LCMC II The Lung Cancer M
“The importance of first-line and second-line targeted agents is obvious”
“The importance of first-line and second-line targeted agents is obvious” Nir P