Enhancing the profile of KRAS-mutant lung cancer
Characteristics and outcomes
KRAS mutations constitute the largest subset of oncogene-driven lung adenocarcinomas, at approximately 30 %. Patients with KRAS-mutant metastatic lung cancer have heterogeneous clinical outcomes depending on the mutation subtype and associated co-mutations. El Osta et al. analysed the Lung Cancer Mutation Consortium (LCMC) database to evaluate the characteristics of these patients and the effect of KRAS mutation features on their outcomes [1]. In all, data from 1,655 patients who consented to participate in LCMC between 2009 and 2015 were analysed for baseline characteristics, mutations status/ subtypes/ co-mutations, OS (calculated from date of distant metastasis to death), and association of patient KRAS data with OS. Median follow-up was 2.15 years.
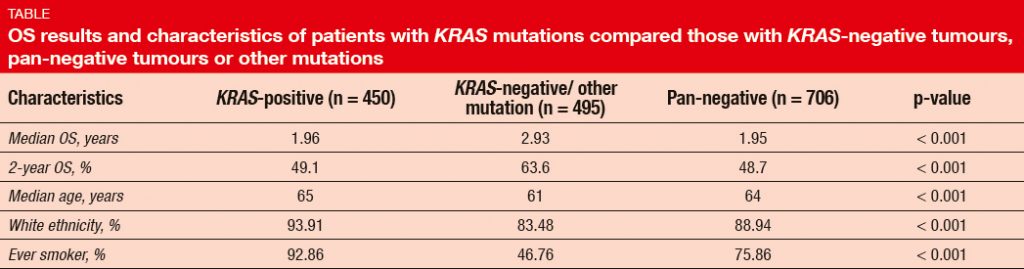
In this population, the incidence of KRAS mutations was 27 %. The presence of KRAS mutation predicted short OS (Table). Compared to patients with other mutations, average patient age was slightly higher in the KRAS-positive cohort, and there was a greater proportion of ever smokers. OS did not differ across KRAS mutations subtypes (i.e., KRAS G12C, G12D, and G12V), with 2-year OS rates of 46.5 %, 47.4 %, and 51.4 %, respectively. However, never smokers were more likely to have KRAS mutant subtype G12D. TP53 mutation occurred as the most common co-mutation (52 %), followed by STK-11 alterations (18 %), MET amplification (4 %), and PIK3CA mutation (3 %). With respect to outcome, STK- 11 co-mutation was shown to be associated with shorter OS.
ntrinsic primary resistance to immunotherapy
Skoulidis et al. retrospectively assessed clinical responses to PD-1/ PD-L1 therapy in co-mutation-defined subsets of KRAS-mutant NSCLC patients [2]. The rationale for this was the fact that the identification of molecular predictors of response to immunotherapy is deemed critical in order to maximise the therapeutic potential of immune checkpoint inhibitors. Previously, patients with KRAS-mutant lung cancer and co-occurring genetic events in STK11/LKB1 or TP53 had been defined as subgroups that showed marked differences in immune contexture. The present cohort included 162 patients harbouring metastatic KRAS-mutant NSCLC who received at least one cycle of PD-1/ PD-L1 therapy (i.e., nivolumab, pembrolizumab, durvalumab, anti-PD-1 plus anti-CTLA-4 therapy) and had available molecular profiling.
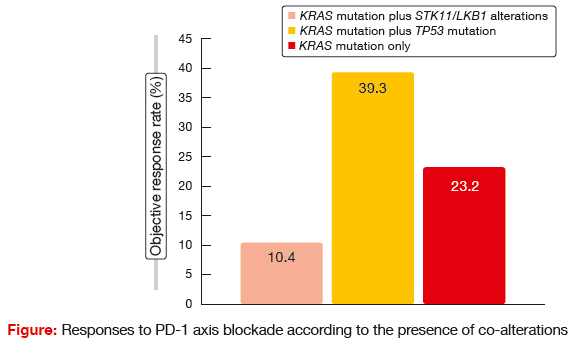
STK11 genetic alterations were demonstrated to be associated with poor response to PD-1 axis blockade. Patients with this co-mutation experienced significantly shorter PFS and OS following PD- 1/ PD-L1 therapy than those with TP53 mutation and KRAS mutation only. There were also significant differences with regard to response rates (Figure). The authors concluded that genetic alterations in the STK11/LKB1 tumour suppressor gene represent a novel, prevalent, tumour- cell-intrinsic mediator of primary resistance to PD-1/ PD-L1 blockade in KRAS-mutant NSCLC. Therefore, in addition to PD-L1 expression and tumour mutational burden, personalised immunotherapy approaches should take the co-mutation status of individual tumours into consideration.
References:
- El Osta B et al., Characteristics and outcomes of patients with metastatic KRAS mutant lung adenocarcinomas: Lung Cancer Mutation Consortium (LCMC) database. ASCO 2017, abstract 9021
- Skoulidis F et al., STK11/LKB1 co-mutations to predict for de novo resistance to PD-1/PD-L1 axis blockade in KRAS-mutant lung adenocarcinoma. ASCO 2017, abstract 9016
More posts
Reducing the danger that arises from the CNS as a site of progression
Reducing the danger that arises from the CNS as a site of progression Brain met
New standards of care for ALK-positive disease
New standards of care for ALK-positive disease The first-generation ALK inhibit
EGFR-targeted treatments: insights from the adjuvant to the resistant setting
EGFR-targeted treatments: insights from the adjuvant to the resistant setting G
Preface – ASCO 2017
Preface – ASCO 2017 Anna Nowak, MBBS FRACP PhD