New standards of care for ALK-positive disease
The first-generation ALK inhibitor crizotinib is the current standard option for patients with newly diagnosed, advanced ALK-positive NSCLC. However, patients invariably relapse on crizotinib treatment, with the central nervous system (CNS) being one of the most common and challenging sites of relapse. The second-generation ALK inhibitor alectinib is more potent than crizotinib [1, 2] and shows clinical activity in crizotinib-resistant NSCLC [3-6]. Notably, trial data have indicated significant CNS activity. Alectinib has become a standard therapy for patients with crizotinib pre-treated ALK-positive NSCLC, but research efforts are ongoing to establish it as a first-line option.
Superiority of alectinib in ALEX
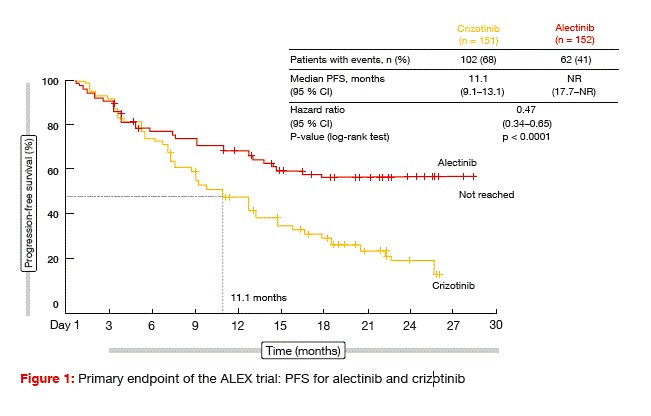
The ALEX trial evaluated alectinib 600 mg twice daily compared to crizotinib 250 mg twice daily in untreated patients with advanced or metastatic ALK-positive NSCLC [7]. ALEX was the first global randomised phase III study to compare a next- generation ALK TKI with a first- generation ALK TKI in the first-line setting. In all, 303 patients were randomised in a 1:1 fashion across 98 sites in 29 countries. Patients with asymptomatic, treated or untreated brain metastases were also enrolled. Approximately 40 % of individuals in each arm harboured CNS metastases, which were untreated in 60 % of cases. Investigator-assessed PFS was defined as the primary endpoint.
The study met its primary objective. At the time of the analysis, median PFS had not been reached in the alectinib- treated arm, while it was 11.1 months in the crizotinib arm (Figure 1). This translated into a risk reduction of 53 % (HR, 0.47; p < 0.0001). PFS according to the Independent Review Committee, which was a secondary endpoint, was also significantly longer with alectinib (25.7 vs. 10.4 months; HR, 0.50; p < 0.0001). Objective responses occurred in 83 % versus 76 % of each arm, although this difference did not reach statistical significance (p = 0.09). However, duration of response was significantly longer with alectinib (not reached vs. 11.1 months; HR, 0.36). Median OS had not been reached in either treatment arm.
Alectinib showed a more favourable AE profile than crizotinib, with lower rates of nausea, diarrhoea, vomiting, peripheral oedema, dysgeusia, transaminase elevations, and visual impairment. The AE profile of alectinib included increased bilirubin levels, myalgia, anaemia, and weight. Dose reductions and treatment discon- tinuations were less frequent in the experimental arm, and the duration of treatment was longer with alectinib than with crizotinib.
Findings on intracranial activity
Almost all of the subgroups derived greater PFS benefit from alectinib than from crizotinib. This implies that patients both with and without brain metastases fared better with the new ALK TKI. For patients with CNS metastases at baseline, median PFS was not reached vs. 7.4 months (HR, 0.40), and for those without CNS metastases at baseline, it was not reached vs. 14.8 months (HR, 0.51).
Time to CNS progression in the total population represented a key secondary endpoint. According to a competing risk analysis with CNS progression, non- CNS progression and death as competing events, the risk of having CNS progression as the first event was reduced by as much as 84 % in the alectinib-treated arm (cause-specific HR, 0.16). At 12 months, only 9.4 % of alectinib-treated patients showed CNS progression, whereas this was 41.4 % in the crizotinib arm. The CNS ORR, as opposed to the overall ORR, demonstrated significant benefit of alectinib therapy. For patients with measurable lesions at baseline, response rates were 81 % vs. 50 % with alectinib and crizotinib, and for those with measurable and non-measurable CNS lesions, 59 % vs. 26 %. Complete remissions occurred in 38 % vs. 5 % for measurable, and 45 % vs. 9 % for non- measurable CNS lesions. The median duration of response in the brain was 17.3 vs. 5.5 months and not reached vs. 3.7 months, respectively.
As the authors summarise, the large magnitude of benefit observed with alectinib suggests that first-line alectinib will be superior to the sequential treatment of crizotinib followed by alectinib. Overall, these results establish alectinib as the new standard of care for patients with previously untreated, advanced, ALK-positive NSCLC.
Third-generation agent lorlatinib
Secondary mutations in the ALK domain can induce resistance to first- generation and second-generation ALK TKIs, which calls for yet other treatment options. The potent third-generation TKI lorlatinib is a selective inhibitor of ALK and ROS1, with broad-spectrum effects against most known ALK resistance mutations, including G1202R [8, 9]. Also, lorlatinib can cross the blood–brain barrier to achieve clinically meaningful CNS activity. Indeed, a phase I trial showed intracranial efficacy for lorlatinib, which included deep responses in patients with measurable disease [10].
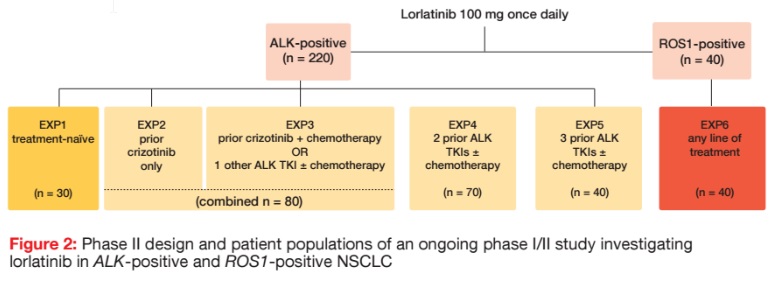
At present, lorlatinib 100 mg/d is being evaluated in an ongoing phase I/ II study in 220 patients with ALK-positive NSCLC and 40 patients with ROS1-positive disease. In the phase II portion of the trial, patients with ALK rearrangement were divided into five expansion cohorts according to their pre-treatment (Figure 2). One cohort was treatment-naïve (EXP1), while groups EXP2 to EXP5 had received prior crizotinib only (EXP2), prior crizotinib plus chemotherapy or one other ALK TKI with or without chemotherapy (EXP3), two prior ALK TKIs with or without chemotherapy (EXP4), or three prior ALK TKIs with or without chemotherapy (EXP5). Patients with ROS1 rearrangement are receiving lorlatinib as any line (EXP6).
The data presented at the ASCO Congress related to groups EXP2 to EXP5; i.e., the ALK-positive cohort that had been treated with at least one ALK TKI prior to study entry [11]. Together, EXP2 and EXP3 included 80 patients, while EXP4 consisted of 70 patients, and EXP5 of 40 patients. Brain metastases were present in 55 % to 71 % of cases across these cohorts at the time of study entry. The primary endpoint was ORR/ intracranial ORR, according to the Independent Review Committee.
Meaningful and durable responses in heavily pre-treated patients
In the total cohort, ORR was 32.9 %. Complete responses occurred in 1.2 %, partial responses in 31.7 %, and disease stabilisation in 32.9 %. At week 12, the DCR was 56.1 %. For cohorts EXP2, EXP3, EXP4 and EXP5, ORRs were 57.1 %, 44.4 %, 25.0 % and 30.8 %, respectively. The majority of patients experienced decrease in target lesion size. There was one complete remission in the EXP4 cohort.
In addition, lorlatinib therapy evoked robust and clinically meaningful intracranial activity, which included complete intracranial responses, irrespective of prior lines of therapy. Target lesions plus non-target lesions together showed ORR of 48.1 %; for target lesions only, this was 51.4 %. Complete responses occurred in 26.9 % and 20.0 % in these two groups. Disease control was 75.0 % at 12 weeks for patients with target lesions plus non-target lesions. With regard to the systemic and intracranial activities, the responses proved durable. At data cut-off, the longest duration of treatment was more than 300 days, and the longest duration of intracranial response was 7 months.
In the entire group incorporating cohorts EXP1 to EXP6, the safety analysis identified hyperlipidaemia as the most common AE, although this was successfully managed with lipid- lowering agents. Cognitive effects occurred in 19.0 % (across all grades) and were generally mild and rapidly reversible upon dose modification. Dose delays became necessary in 29.3 %, and dose reductions in 19.8 %. Only 3.4 % of patients discontinued treatment due to AEs. Based on these data, lorlatinib has received Breakthrough Therapy designation from the US Food and Drug Administration for use in patients with ALK-positive metastatic NSCLC previously treated with at least one ALK TKI. The phase III CROWN study, which is comparing first-line lorlatinib to crizotinib, is presently recruiting patients.
Analysis of EML4-ALK variants
Ou et al. investigated the association of ALK resistance mutations with specific variants of the EML4-ALK rearrangement [12]. Samples from 634 patients with ALK-positive NSCLC collected in the FoundationCORE database were analysed. The most common variants were EML4-ALK v1 and EML4-ALK v3a/b, each of which was found in 32 % of cases. EML4-ALK v2 occurred in 8 %, other EML4-ALK variants in 12 %, and non-EML4-ALK rearrangements in 16 %. The presence of known ALK resistance mutations was significantly associated with v3 as compared to v1 (p = 0.0002). G1202R was the most frequent ALK resistance mutation in this dataset. This mutation also showed significant association with v3 compared to all non-v3 variants (p = 0.0004). Drop-out, switching, and evolution of multiple ALK resistance mutations occurred over the course of sequential ALK inhibitor treatment.
The authors concluded that the use of tissue-based and blood-based next generation sequencing allows for detection of specific ALKfusion variants and increases the understanding of the biology of ALK-positive NSCLC. In addition, it might have value to predict potential mechanisms of resistance and inform the selection of ALK inhibitor therapy. Non-ALK mechanisms of acquired resistance should be considered, especially in tumours with ALKrearrangements that are non- variant 3. For instance, MET kinase domain duplication was identified as a novel mechanism of acquired resistance after crizotinib and ceritinib treatment in a patient harbouring EML4-ALK v1.
References:
- Sakamoto et al., CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011; 19: 679- 690
- Kodama et al., Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 2014; 351: 215-221
- Ou et al., Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016; 34: 661-668
- Shaw et al., Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016; 17: 234-242
- Yang JC et al., Pooled efficacy and safety data from two phase II studies (NP28673 and NP28761) of alectinib in ALK+ non-small-cell lung cancer (NSCLC). WCLC 2016, abstract P3.02a-016 REFERENCES
- Gadgeel et al., Pooled Analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small- cell lung cancer. J Clin Oncol 2016; 34: 4079- 4085
- Shaw AT et al., Alectinib vs. crizotinib in treatment-naïve advanced ALK+ NSCLC: primary results of the global phase III ALEX study. ASCO 2017, abstract LBA9008
- Gainor JF et al., Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016; 6: 1118-1133
- Johnson TW et al., Discovery of (10R)-7- amino-12-fluoro-2,10,16-trimethyl-15-oxo- 10,15,16,17-tetrahydro-2H-8,4-(metheno) pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetra- decine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 2014; 57: 4720-4744
- Solomon BJ et al., Safety and efficacy of lorlatinib (PF-06463922) from the dose- escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). J Clin Oncol 2016; 34 (suppl; abstr 9009)
- Shaw AT et al., Efficacy and safety of lorlatinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) with one or more prior ALK tyrosine kinase inhibitor (TKI): A phase I/II study. ASCO 2017, abstract 9006
- Ou S-H I et al., Association of ALK resistance mutations by EML4-ALK variant (v3 vs. non-v3) in ALK+ non-small cell lung cancer (NSCLC). ASCO 2017, abstract 9010
More posts
Reducing the danger that arises from the CNS as a site of progression
Reducing the danger that arises from the CNS as a site of progression Brain met
New standards of care for ALK-positive disease
New standards of care for ALK-positive disease The first-generation ALK inhibit
EGFR-targeted treatments: insights from the adjuvant to the resistant setting
EGFR-targeted treatments: insights from the adjuvant to the resistant setting G
Preface – ASCO 2017
Preface – ASCO 2017 Anna Nowak, MBBS FRACP PhD