New insights into the treatment of ALK-mutant-positive NSCLC patients
Anaplastic lymphoma kinase (ALK) is a fusion oncogene, and the prevalence of ALK mutations in NSCLC patients is similar across different races. At CSCO 2017, the main progress for the treatment of ALK-mutant-positive NSCLC patients related to the new recommendations for first-line and second-line treatments, and the optimal strategies to manage patients before and after resistance to ALK tyrosine kinase inhibitors (TKIs). Strategies for the management of patients with concomitant EGFR-ALK mutations were also reported.
First-line treatment of ALK-mutant-positive NSCLC patients
Crizotinib is recommended by CSCO as first-line treatment of ALK-mutant-positive NSCLC patients. However, the recently published J-ALEX and ALEX studies that compared alectinib to crizotinib as first-line treatments for these patients showed improved progression-free survival for alectinib compared to crizotinib [1]. Thus, the NCCN guidelines for NSCLC recommend alectinib as first-line treatment for ALK-mutant-positive NSCLC patients [2].
Based on the results of the J-ALEX and ALEX trials, Professor Jie Wang Key from the Department of Thoracic Medical Oncology, Peking University Cancer Hospital and Institute, Beijing (China) indicated that although alectinib has already been shown to be more effective than crizotinib, the currently ongoing Phase III studies on other ALK inhibitors still use crizotinib as first-line treatment for the control group; i.e., the ALTA-1L trial with brigatinib, the CROWN trial with lorlatinib, and the eXalt3 trial with ensartinib. Thus, whether this set-up with crizotinib as the control first-line treatment is appropriate is now questionable [3].
Second-line treatment of ALK-mutant-positive NSCLC: a new breakthrough?
Acquired resistance is the major limitation of ALK TKIs as first-line treatments in clinical practice. Roughly 30% of crizotinib-treated refractory tumors have been shown to have resistance mutations in the ALK kinase domain, which include G1269A, L1196M, C1156Y, L1152R, S1206Y, 1151Tins, G1202R, and F1174L [4]. Lorlatinib is a third-generation ALK TKI that has shown efficacy against most resistance mutations in preclinical studies [5]. While these data are promising, they still need to be confirmed in clinical studies in ALK-mutant-positive NSCLC patients with resistance to first-line treatments.
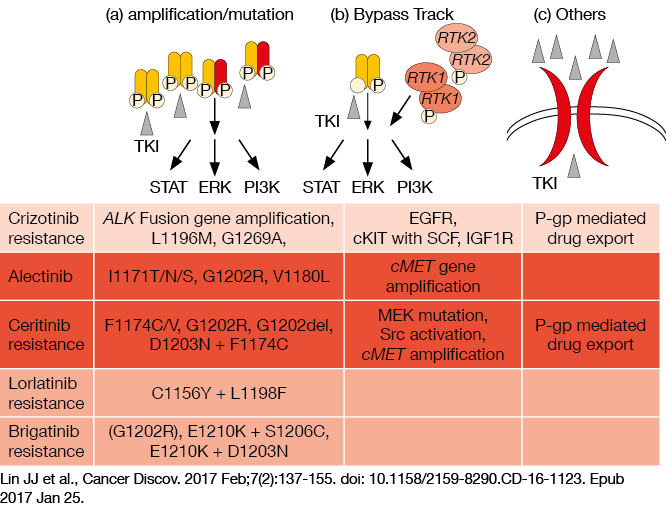
Resistance mechanisms to ALK TKIs: investigations and findings
There are several studies that are exploring the mechanisms of resistance to crizotinib, while there remain few such studies for second-generation and third-generation ALK TKIs. However, at CSCO 2017, Professor Wang indicated that there appear to be three main mechanisms of resistance to second-generation and third-generation ALK TKIs: (i) amplification/ mutation, such as G1202R for alectinib resistance, F1174C/V for ceritinib reistance, C1156Y+L1198F for lorlatinib resistance, and G1202R for brigatinib resistance; (ii) bypass track, such as cMET gene amplification for alectinib resistance and MEK mutation for ceritinib resistance; and (iii) other mechanisms, such as P-gp–mediated drug export (Figure 1). Thus, Professor Wang said that combination therapy stategies might be beneficial to prevent bypass-activating resistance, such as combinations with EGFR TKIs, to prevent resistance arising through EGFR bypass pathways.
Figure 1: Illustration of the three main mechanisms of resistance to second-generation and third-generation ALK TKIs
Heterogeneous diagnosis and treatment of ALK-positive NSCLC
Concomitant mutations have been observed in NSCLC patients, with the prevalence of EGFR–ALK concomitant mutations at about 0.1 % [6]. Lou et al. [7] reported that first-line treatment of EGFR-ALK-mutant NSCLC patients with EGFR TKIs provided better outcomes compared to chemotherapy, crizotinib or vascular endothelial growth factor receptor TKIs. However, a study by Lo Russo G et al. [8] reported that for NSCLC patients with co-occurrence of EML4-ALK rearrangement and EGFR mutations, the ALK TKI provided greater complete/ partial responses than the EGFR TKI (51.3 % vs. 43.4 %). The authors indicated that the discrepancy between Lou et al. [7] and Lo Russo G et al. [8] might be due to heterogeneous patterns of concomitant ALK/EGFR mutations [9].
REFERENCES
- Peters S et al., Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2017;377(9):829-838.
- NCCN Clinical Practice Guidelines in Oncology: Non–Small Cell Lung Cancer; version 7, 2017.
- Jie W et al., Treatment Strategy for Advanced ALK-Positive NSCLC. CSCO 2017.
- Gainor JF et al., Emerging Paradigms in the Development of Resistance to Tyrosine Kinase Inhibitors in Lung Cancer. J Clin Oncol. 2013;31:3987-3996.
- Zou HY et al., PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell. 2015;28(1):70-81.
- Ulivi P et al., Nonsquamous, Non–Small-Cell Lung Cancer Patients Who Carry a Double Mutation of EGFR, EML4-ALK or KRAS: Frequency, Clinical-Pathological Characteristics, and Response to Therapy. Clin Lung Cancer. 2016;17(5):384-390.
- Lou NN et al., Clinical Outcomes of Advanced Non–Small-Cell Lung Cancer Patients with EGFR Mutation, ALK Rearrangement and EGFR/ALK Co-Alterations. Oncotarget. 2016;7(40):65185-65195.
- Lo Russo G et al., Concomitant EML4-ALK Rearrangement and EGFR Mutation in Non–Small Cell Lung Cancer Patients: a Literature Review of 100 Cases. Oncotarget. 2017;8(35):59889-59900.
- Cai W et al., Intratumoral Heterogeneity of ALK-Rearranged and ALK/EGFR Coaltered Lung Adenocarcinoma. J Clin Oncol. 2015;33(32):3701-3709.