Reaching unprecedented outcome dimensions in malignant mesothelioma
Malignant pleural mesothelioma (MPM) is a rare but aggressive cancer with poor prognosis. While combination chemotherapy with platinum and pemetrexed with or without bevacizumab is a standard in first-line treatment, no approved second-line strategies have been established to date [1]. Gemcitabine or vinorelbine are often used in this situation, but these only show limited activity [2].
However, there is a strong rationale for the assessment of immunotherapy in patients with MPM. The inflammatory phenotype of these tumours hints at the involvement of T cells, and MPM cells express PD-L1 in a substantial proportion of cases [3-6]. Moreover, PD-L1 expression has been correlated with worse prognosis in MPM [7, 8].
MAPS2: combination immunotherapy
The randomised, non-comparative phase II MAPS-2 trial independently evaluated nivolumab 3 mg/kg every 2 weeks (n = 63) and the combination of nivolumab with the anti-CTLA-4 antibody ipilimumab 1 mg/kg every 6 weeks (n = 62) until disease progression or toxicity, for a maximum of 2 years. Patients with unresectable MPM and documented progression after one or two lines of chemotherapy including a pemetrexed/platinum doublet were enrolled. In each arm, PD-L1 expression status was available in 79 % of patients.
The disease control rate (DCR) at 12 weeks was defined as the primary endpoint based on the statistical plan and was met in both arms. Among the first 108 eligible patients, 50.0 % and 44.4 % treated with the combination and nivolumab monotherapy, respectively, experienced disease control at 12 weeks, as previously reported [9]. In the ITT population, DCRs amounted to 51.6 % and 39.7 %, respectively. These are meaningful increases compared to historical data and previous non-immunotherapy clinical trials.
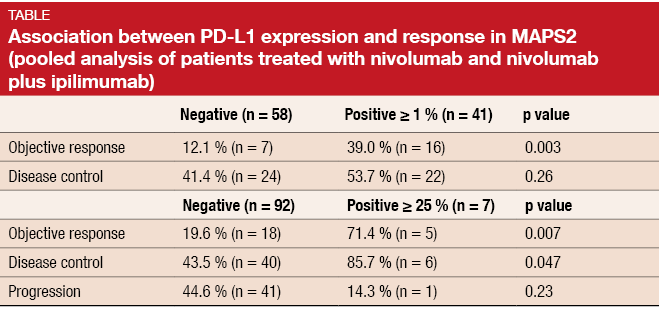
Zalcman et al. presented updated findings from the MAPS-2 trial at the ESMO 2017 Congress [10]. According to a pooled analysis of patients with available PD-L1 status, PD-L1 expression ≥ 1 % significantly correlated with response, and high PD-L1 expression (≥ 25 %) correlated with both objective response and disease control (Table). Median duration of response was 7.9 and 7.4 months, respectively. Long-lasting remissions were observed for all histological subtypes (i.e., epithelioid, biphasic, sarcomatoid).
Extension of median survival beyond 15 months
After a median follow-up of 15 months, median OS was not reached and 13.6 months in the combination and monotherapy arms, respectively. As in the most recent analysis [9], PFS for nivolumab plus ipilimumab and nivolumab alone was 5.6 and 4.0 months, respectively, after the extended follow-up showing the maturity of such analyses. The exploratory forest plot showed that patients with sarcomatoid/ biphasic histology fared better regarding OS when they were treated with the combination, while they did worse with nivolumab monotherapy. This also applied to those who received immunotherapy in the third versus second line. Conversely, patients with PD-L1 expression (≥ 1 % vs. < 1 %) benefited from nivolumab, while both subsets benefited equally from nivolumab plus ipilimumab. The greatest benefit from nivolumab only occurred in patients who had progressed more than 3 months after pemetrexed therapy (HR, 0.25; p = 0.002). Due to small patient numbers, these results are only hypothesis-generating, however.
Toxicity of the regimens assessed was generally manageable. Grade 3 AEs occurred more frequently with the combination, although not to a significant degree (22.9 % vs. 12.7 %). With nivolumab plus ipilimumab, two patients experienced grade 4 AEs, and there were three deaths deemed treatment-related, occurring early in the trial course, due to fulminant hepatitis, encephalitis, and acute kidney failure. None of the patients receiving nivolumab monotherapy had grade 4/5 AEs. Patients in the combination arm reported more frequently diarrhoea, pruritus, and dry skin. For the majority of documented immune-related AEs, higher rates were noted with nivolumab plus ipilimumab, but most of these were grades 1 and 2.
Quality of life assessments at 12 weeks favoured nivolumab monotherapy for global, pain, anorexia and interference items, although not significantly. On the other hand, patients treated with the combination reported advantages regarding the general item and symptom distress scales. Long-term and longitudinal quality-of-life studies are pending. As the authors concluded, the results of MAPS-2 support the recent NCCN panel decision to recommend the monotherapy or the combination therapy as options for the second or third line in relapsing MPM patients.
Activity of pembrolizumab in a Swiss registry
Early phase trials investigating the anti-PD-1 antibody pembrolizumab in patients with mesothelioma have yielded promising outcomes. In the KEYNOTE-028 study, DCR was 72 %, and median OS amounted to 18 months [11]. The Chicago cohort showed a DCR of 80 % and a median OS of 11.9 months [12]. Based on these trials, pembrolizumab started to be used for the off-label treatment of relapsed MPM in Switzerland. The aim of the Swiss registry was to assess the activity of pembrolizumab in relapsed MPM in a real-life setting. Thirteen cancer centres in Switzerland contributed their data. PD-L1 quantification was performed in a central laboratory, whereas local investigators determined clinical responses.
According to a retrospective analysis of the registry, 48 patients with a median age of 68.5 years at diagnosis were included until April 2017 [13]. The majority (73 %) had tumours with epithelioid histology. In 10 %, histology was sarcomatoid, and in 17 %, it was mixed. Virtually all patients had received prior chemotherapy. The pembrolizumab doses ranged from 2 mg/kg every 3 weeks to 10 mg/kg every 2 weeks. Most patients received pembrolizumab at a flat dose of 200 mg every 3 weeks.
Outcomes according to PD-L1 expression
Among the 48 patients included in this analysis, one and 11 achieved CR and PR, respectively, which added up to an ORR of 25 %. This was similar to early clinical trial data with PD-(L)-1 inhibitors [11, 12, 14] and compared favourably to current chemotherapy options. With 13 additional patients achieving SD, DCR was 52 %. The median PFS and OS in the entire cohort were 3.6 months and 7.2 months, respectively. Predictors of improved survival with pembrolizumab included good performance status, early line of treatment, and sarcomatoid histology. Survival results obtained in these selected groups resembled those from the KEYNOTE-028 trial and the Chicago cohort [11, 12]. On the other hand, the outcomes in the all-comer population included in this registry were clearly inferior to those observed in the trials.
With regard to PD-L1 expression, results were available for 37 patients. Sixty-seven percent of these were PD-L1–negative by definition (i.e., PD-L1 expression < 5 %), while 22 % and 11 % had PD-L1 expression of 5-49 % and ≥ 50 %, respectively. A significant correlation between histology and PD-L1 expression was found, as PD-L1 negativity prevailed in epithelioid tumours, while high PD-L1 expression correlated with sarcomatoid histology. The PD-L1-positive subgroups showed 4-5 fold higher ORRs compared to the PD-L1–negative cohort. Patients with PD-L1 expression ≥ 50 % achieved a DCR of 100 %. Likewise, PFS and OS improved with increasing PD-L1 expression. Of note, the single patient who achieved complete remission had both sarcomatoid histology and high PD-L1 expression. The authors concluded that these two features might be predictive for improved outcomes with pembrolizumab treatment.
Fifteen treatment-related AEs occurred in 14 patients, with five grade 3/4 AEs, four of which had resolved at the time of data cut-off. Seven patients (15 %) discontinued pembrolizumab treatment due to AEs. An ongoing prospective randomised controlled trial will establish the role of checkpoint inhibition in MPM.
First-line benefit from nintedanib treatment
The oral multikinase inhibitor nintedanib is being investigated in patients with unresected MPM in the randomised, double-blind, placebo-controlled phase II/III LUME-Meso trial. Chemotherapy-naïve patients are treated with either nintedanib 200 mg twice daily plus pemetrexed/cisplatin (n = 44) or placebo plus pemetrexed/cisplatin (n = 43). Patients in the experimental arm without disease progression receive nintedanib maintenance. At the ESMO 2017 Congress, mature OS and forced vital capacity (FVC) results from the phase II part of the trial were reported [15].
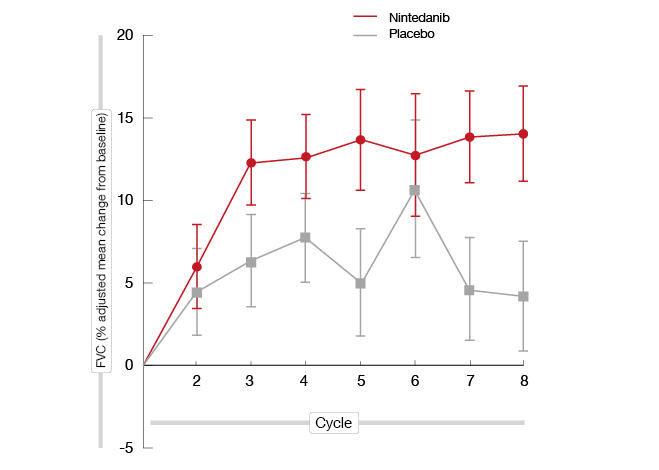
A trend towards improvement in OS favouring nintedanib treatment became evident for the whole cohort (18.3 vs. 14.2 months; HR, 0.77; p = 0.3193). The survival benefit conferred by nintedanib treatment was greatest in patients with epithelioid histology (20.6 vs. 15.2 months; HR, 0.70; p = 0.1965). FVC was included as an endpoint because it reflects patient performance and quality of life in MPM. Higher baseline FCV and increases in FVC during treatment correlate with better patient-reported outcomes [16, 17]. Indeed, according to this analysis, adjusted mean percentage change in FVC from baseline favoured nintedanib over placebo from cycle 2 for all patients and those with epithelioid histology (Figure). The same was true at cycle 8; here, the mean treatment difference was 7.2 % for all patients and 9.9 % for the group with epithelioid histology.
Figure: Adjusted mean percentage in FVC from baseline for patients with epithelioid histology who received either nintedanib or placebo
Confirmation of the primary PFS analysis
As for the initial analysis, updated PFS data showed that nintedanib treatment improved PFS compared with placebo (9.4 vs. 5.7 months; HR, 0.54; p = 0.0103). This improvement was greatest in patients with epithelioid histology (9.7 vs. 5.7 months; HR, 0.49; p = 0.0056). Patients in the nintedanib arm developed numerically more objective responses than those in the placebo arm (56.8 % vs. 44.2 %). All of these were partial responses.
The safety profile of nintedanib treatment proved manageable and consistent with previous studies. AEs commonly associated with anti-angiogenic agents were either balanced between treatment arms or reported in fewer patients in the nintedanib arm than in the control arm. In addition, AEs leading to permanent study discontinuation of the last study medication occurred less frequently with nintedanib than with placebo (6.8 % vs. 17.1 %). Nintedanib did not compromise delivery of the backbone chemotherapy. The phase III part of the LUME-Meso study is currently recruiting patients with epithelioid histology.
References
- Baas P et al., Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26 Suppl 5: v31-v39
- Zauderer mg et al., Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer 2014; 84(3): 271-274
- Thapa B et al., The immune microenvironment, genome-wide copy number aberrations, and survival in mesothelioma. J Thorac Oncol 2017; 12(5): 850-859
- Lanteajoul S et al., PD-L1 Testing for Immune checkpoint inhibitors in mesothelioma: for want of anything better? J Thorac Oncol 2017; 12(5): 778-778
- Mansfield AS et al., B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 2014; 9(7): 1036-1040
- Khanna S et al., Malignant mesothelioma effusions are infiltrated by CD3+ T cells highly expressing PD-L1 and the PD-L1+ tumor cells within these effusions are susceptible to ADCC by the anti-PD-L1 antibody avelumab. J Thorac Oncol 2016; 11(11): 1993-2005
- Cedrés S et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015; 10(3): e0121071
- Combaz-Lair C et al., Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum Pathol 2016; 52: 9-18
- Scherpereel A et al., Second or third-line nivolumab versus nivolumab plus ipilimumab in malignant pleural mesothelioma patients: results of the IFCT-1501 MAPS-2 randomized phase 3 trial. ASCO 2017, abstract LBA8507
- Zalcman G et al., Second or 3rd line nivolumab (nivo) or nivo plus ipilimumab in malignant pleural mesothelioma (MPM) patients: up-dated results of the IFCT-1501 MAPS1 randomized phase 2 trial. ESMO 2017, abstract LBA58_PR
- Alley E et al., Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017; 18: 623-630
- Kindler H et al., Phase II trial of pembrolizumab in patients with malignant mesothelioma (MM): interim analysis. WCLC 2016, abstract OA13.02
- Mauti LA et al., Pembrolizumab as second or further line treatment in relapsed malignant pleural mesothelioma; a Swiss registry. ESMO 2017, abstract 1615O
- Quispel-Janssen J et al., A phase II study of nivolumab in malignant pleural mesothelioma NivoMes): with translational research (TR) biopsies. WCLC 2016, OA13.01
- Novello S et al., Overall survival and forced vital capacity results from the LUME-Meso study of nintedanib + pemetrexed/cisplatin versus placebo + pemetrexed/ciusplatin in chemotherapy-naïve patients with malignant pleural mesothelioma. ESMO 2017, abstract 1618PD
- Krug LM et al., Forced vital capacity (FVC) as a reproducible measure of pulmonary function (PF) in chemotherapy-pretreated patients with malignant pleural mesothelioma (MPM). J Clin Oncol 29: 2011 (suppl; abstr 7028)
- Nowak AK et al., Assessing quality of life during chemotherapy for pleural mesothelioma: feasibility, validity, and results of using the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire and Lung Cancer Module. J Clin Oncol 2004; 22: 3172-3180
More posts
Immunostimulation as a promising approach in SCLC
Immunostimulation as a promising approach in SCLC IMPULSE There is a high unmet
Immunotherapy: once more at the cutting edge of progress
Immunotherapy: once more at the cutting edge of progress PACIFIC: durvalumab in
Preface – ESMO 2017
Preface – ESMO 2017 David R. Gandara, MD Professor of Medicine UC Davis Compreh