Potent treatment options in ALK– and MET-positive disease
ALESIA: confirming findings obtained in ALEX
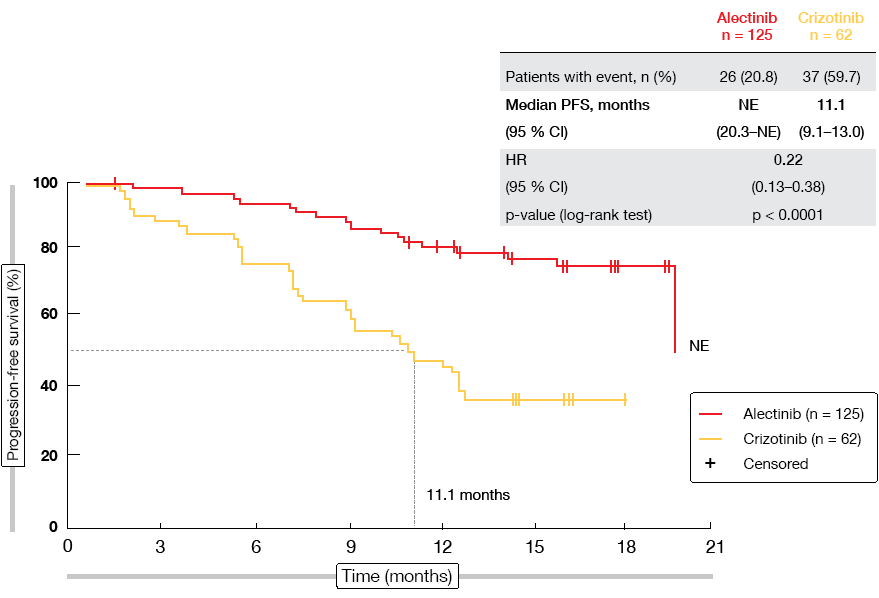
The highly selective, CNS-active ALK inhibitor alectinib has demonstrated superiority over crizotinib in the first-line setting of ALK-positive NSCLC both in the global phase III ALEX study [1] and the phase III J-ALEX trial, which was conducted in Japanese patients [2]. Alectinib has been approved in the US and Europe and has recently received priority approval in China. At the ESMO 2018 Congress, Zhou et al. reported the primary results from the phase III ALESIA study that evaluated first-line alectinib compared to crizotinib in Asian patients with advanced ALK-positive NSCLC using the globally approved alectinib dose [3]. The primary objective of the study was the proof of consistency with the PFS benefit of alectinib observed in the ALEX trial. Consistency was defined as maintaining ≥ 50 % of the risk reduction observed in ALEX, where PFS amounted to 34.8 months for alectinib versus 10.9 months for crizotinib (HR, 0.43) [1]. A total of 187 patients were randomised at 21 sites in China, South Korea, and Thailand. In the experimental arm, 125 patients received alectinib 600 mg twice daily, while 62 patients in the control arm were treated with crizotinib 250 mg twice daily. PFS as determined by the investigators constituted the primary endpoint.
ALESIA did meet its primary outcome, thus further confirming alectinib as a standard of care in the first-line treatment of ALK-positive NSCLC. PFS was significantly improved compared to crizotinib according to the investigators (not reached vs. 11.1 months; HR, 0.22; p < 0.0001; Figure 1). This was also true for PFS according to independent review committee (not reached vs. 10.7 months; HR, 0.37; p < 0.0001), which was defined as a secondary endpoint. All of the subgroups favoured alectinib treatment. Additional benefits were noted for ORR (91.2 % vs. 77.4 %) and duration of response (not reached vs. 9.3 months; HR, 0.22; p < 0.0001). Intracranial response rates obtained with alectinib exceeded those with crizotinib in both patients with measurable baseline CNS lesions (94.1 % vs. 28.6 %) and those with both measurable and non-measurable brain metastases (72.7 % vs. 21.7 %). According to a competing risk analysis, CNS progression without prior non-CNS progression or death was significantly lower with alectinib than with crizotinib (7.3 % vs. 35.5 %; cause-specific HR, 0.14; p < 0.0001). OS data were still immature. The safety results in this Asian population generally matched the known safety profile of alectinib.
Figure 1: Primary endpoint of the ALESIA trial: progression-free survival according to investigator
Intracranial effects of brigatinib
Another option that has shown convincing results in ALK-inhibitor–naïve patients is the next-generation ALK/ROS1 inhibitor brigatinib. Compared to crizotinib, brigatinib gave rise to superior PFS in the open-label, randomised, phase III ALTA-1L study at the first planned interim analysis (HR, 0.49; p = 0.0007) [4]. Detailed intracranial efficacy data from this analysis were reported at the ESMO Congress [5]. At baseline, one third of patients in both treatment arms showed CNS lesions.
In patients with baseline brain metastases, whole-body PFS HR was 0.20 and therefore one of the lowest HRs noted among subgroups analysed. For those without CNS lesions, the PFS HR did not reach significance presumably due to short follow-up, which might preferentially emphasise CNS progression among patients with brain lesions as an earlier differentiating event. Brigatinib significantly improved confirmed intracranial ORR in both patients with measurable brain metastases at baseline (78 % vs. 29 %; OR, 10.42; p = 0.0028) and those with any brain metastases at baseline (67 % vs. 17 %; OR, 13.00; p < 0.0001). Intracranial PFS was significantly longer with brigatinib than with crizotinib in the intent-to-treat (ITT) population (HR, 0.42; p = 0.0006) and the group with baseline brain metastases (HR, 0.27; p < 0.0001). In those without brain metastases at baseline, intracranial PFS was immature.
An exploratory competing risk analysis was performed to estimate the cumulative incidence for CNS progression, systemic progression, and death by treatment group in the ITT population. This analysis revealed that the treatment with brigatinib significantly delayed both time to CNS progression (without prior systemic progression) and time to systemic progression (without prior CNS progression) compared with crizotinib.
Final results from ASCEND-3
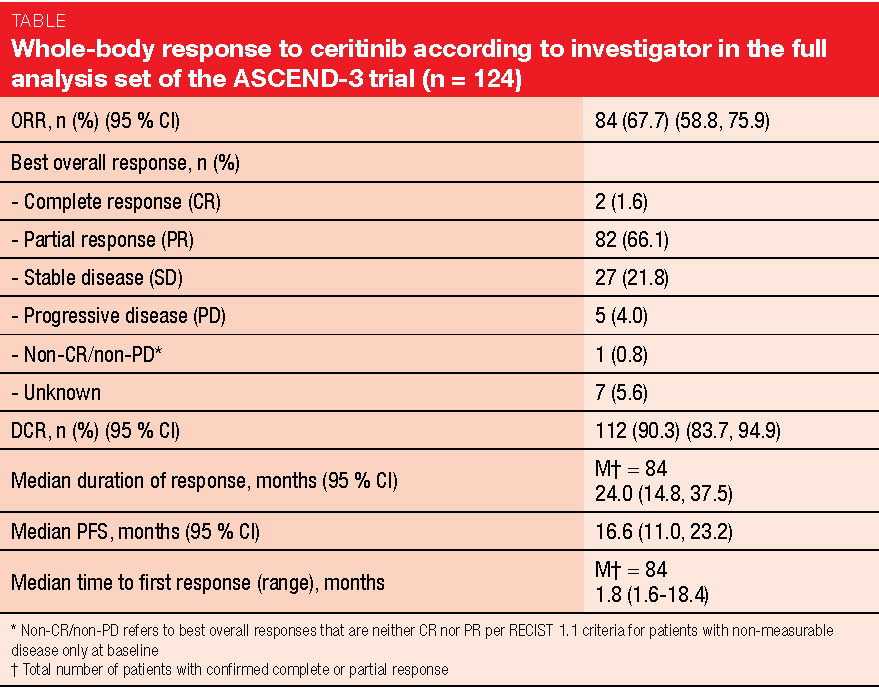
In similar vein, the final efficacy and safety analysis of the single-arm, multicentre phase II ASCEND-3 trial confirmed the positive benefit-risk profile of the next-generation ALK inhibitor ceritinib in ALK-positive NSCLC [6]. ASCEND-3 has already demonstrated clinically relevant ORR (67.6 %) and PFS (16.6 months) results with ceritinib 750 mg/day in 124 ALK-naïve patients who had received ≤ 3 prior lines of chemotherapy and had progressed during or after the last chemotherapy regimen [7]. Asymptomatic or neurologically stable brain metastases at baseline were permitted. In total, 49 patients (39.5 %) presented with CNS lesions at study entry.
After a median follow-up of 52.1 months, ORR according to investigator, which constituted the primary endpoint, was 67.7 % (Table). Disease control occurred in 90.3 %. Responses lasted for a median of 24.0 months. The presence of brain metastases did not affect outcomes; for patients with and without brain lesions, disease control rate was 87.8 % and 92.0 %, respectively. In the overall group, median PFS and OS amounted to 16.6 and 51.3 months, respectively.
The authors summarised that ceritinib gave rise to a clinically relevant prolonged OS outcome in a heavily pre-treated population, as 55 % of patients had received ≥ 2 prior antineoplastic regimens. At 18 months, 65.5 % and 78.4 % of patients with and without brain metastases, respectively, were alive. No new safety signals occurred, and the safety profile was consistent with the known data. The study included an analysis of patient-reported outcomes, according to which qualify of life was generally maintained during ceritinib treatment.
Intake of ceritinib: fed or fasted?
Ceritinib was initially approved at the recommended dose of 750 mg/day fasted for the treatment of ALK-positive NSCLC in the first line or after crizotinib failure. The randomised, open-label phase I ASCEND-8 trial tested ceritinib at three different doses, with 450 mg/day and 600 mg/day administered together with a low-fat meal and 750 mg/day administered under fasted conditions. After this study had yielded similar steady-state exposure and a more favourable gastrointestinal safety profile in the 450 mg fed arm compared to the 750 mg fasted arm, the recommended starting dose of ceritinib was changed to 450 mg/day with food in the US, European Union, and other countries worldwide [8]. Cho et al. presented the primary efficacy findings in treatment-naïve patients and the updated safety in the overall population treated with ceritinib 450 mg (n = 108) or 600 mg (n = 87) with food compared to 750 mg fasted (n = 111) [9].
The results confirmed that ceritinib 450 mg with food shows similar pharmacokinetics, efficacy and a more favourable gastrointestinal safety profile than 750 mg fasted. ORRs with 450 mg fed, 600 mg fed and 750 mg fasted were 78.1 %, 72.5 %, and 75.7 %, respectively. Median PFS was not estimable, 17.0 months, and 12.2 months, respectively. During the on-treatment period, the 450 mg arm showed the highest median relative dose intensity and the lowest proportion of patients with dose reductions among the three treatment arms. Fewer patients treated with 450 mg experienced gastrointestinal toxicities of all grades when compared to the 750 mg arm.
GEOMETRY: capmatinib
METexon-14 skipping mutations represent a novel oncogenic driver and occur in 3 % to 4 % of NSCLC cases [10-12]. They have been recognised as a poor prognostic factor in advanced disease [13]. Preliminary data indicated poor response to standard therapies including immunotherapy, even in the setting of pronounced PD-L1 expression and mutation load [14, 15]. The orally available, highly selective, reversible MET inhibitor capmatinib (INC280) has been shown to be the most potent inhibitor of METΔexon-14 [16]. Capmatinib also crosses the blood-brain-barrier, and preliminary brain activity has been reported [17, 18].
The phase II GEOMETRY mono-1 trial investigated capmatinib 400 mg twice daily in patients with stage IIIB/IV NSCLC with MET-amplification and/or METΔexon-14 mutation [19]. These aberration subtypes were analysed separately, which also applied to pre-treatment status (i. e., treatment-naïve patients; pre-treated patients after one or two lines). Patients with neurologically stable or asymptomatic brain metastases were allowed to enter the trial.
In the METΔexon-14 mutation population, Cohorts 4 (n = 69) and 5b (n = 28) consisted of pre-treated and treatment-naïve patients, respectively. At the ESMO 2018 Congress, Wolf et al. reported the results obtained in these two cohorts that are fully enrolled and closed [19]. ORR by blinded independent review committee (BIRC) constituted the primary endpoint. Each cohort was analysed separately.
Impressive activity in treatment-naïve patients
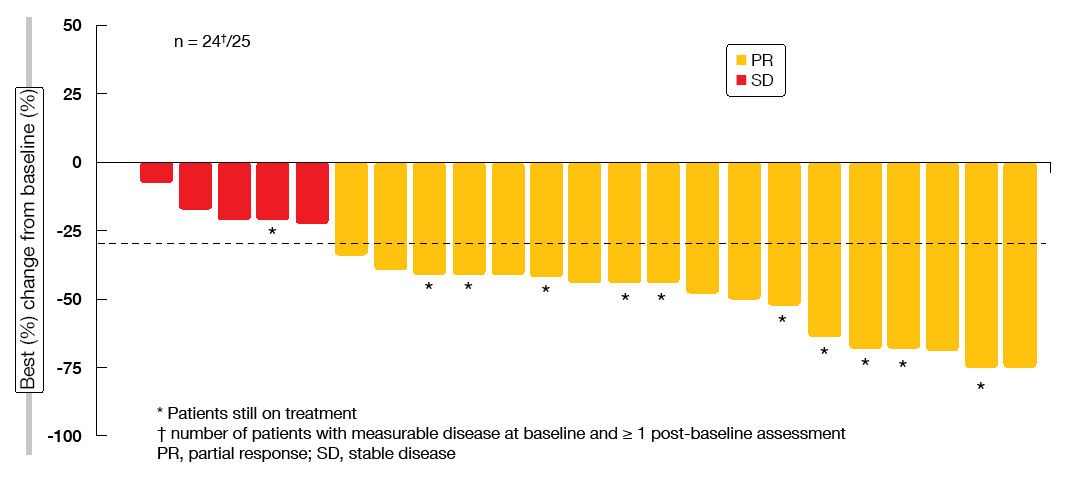
For the pre-treated Cohort 4, ORR according to BIRC was 39.1 %, with disease control occurring 78.3 %. In the treatment-naïve patients from Cohort 5b, these were 72.0 % and 96.0 %, respectively. This suggests almost complete tumour control conferred by capmatinib in the first-line setting. Median duration of response could not be reported for both cohorts, as this outcome was not mature yet. Particularly in the treatment-naïve cohort, most of the patients showed deep responses (Figure 2). Preliminary activity in the brain was demonstrated as exemplified by the case of 80-year-old patient who had multiple untreated CNS metastases at study entry. The patient achieved complete resolution of the brain lesions at the first post-baseline CT scan. She responded for 11.3 months and finally discontinued capmatinib treatment due to extra-cranial progressive disease.
The most common side effects of capmatinib treatment included peripheral oedema, nausea, increases in blood creatinine levels, diarrhoea, decreased appetite, and fatigue. Increases in creatinine levels do not suggest impaired renal function, but rather reduced function of the serum creatinine transporter. Overall, 10.3 % of patients discontinued treatment due to toxicity suspected to be related to capmatinib.
In their conclusion, the investigators pointed out that capmatinib has demonstrated a clinically meaningful response rate and manageable toxicity profile in patients with METΔexon-14–mutated, advanced NSCLC regardless of the line of therapy. The differential benefit observed between patients treated in the first line on one hand and the second or third line on the other highlight the need of early diagnosis and prompt targeted treatment.
Figure 2: Tumour responses to capmatinib in the treatment-naïve cohort by blinded independent review committee
Tepotinib plus gefitinib in MET-/EGFR-positive NSCLC
Dual MET and EGFR inhibition is thought to have therapeutic potential in patients with EGFR-TKI–resistant NSCLC [20]. The orally available, highly selective MET TKI tepotinib was shown to be able to overcome acquired resistance to EGFR TKIs due to aberrant MET activation in preclinical models [21]. At the ESMO 2018 Congress, randomised phase II data were presented from a phase Ib/II trial of tepotinib plus gefitinib in patients with relapsed EGFR-mutant, MET-positive NSCLC [22]. The open-label, single-arm, phase Ib dose escalation part had established tepotinib 500 mg/day in combination with gefitinib 250 mg/day as the recommended phase II dose [23]. In the phase II part, Asian patients with EGFR-positive, T790M-negative, locally advanced or metastatic NSCLC that showed MET overexpression (MET2+ or 3+ by immunohistochemistry) and/or MET amplification by in-situ hybridisation received either tepotinib 500 mg/day plus gefitinib 250 mg/day or pemetrexed plus cisplatin and carboplatin. Resistance to prior EGFR TKI treatment was an inclusion criterion. The patients had not received any prior HGF/MET-pathway–directed therapy. Overall, 55 patients were identified 31 of whom received the experimental treatment. Enrolment was halted early due to low recruitment.
Progression-free survival according to the investigators did not differ between the two treatment arms (4.9 vs. 4.4 months with tepotinib plus gefitinib and chemotherapy, respectively; HR, 0.71). However, in the group of patients with high MET expression (IHC3+), PFS was almost double in the experimental arm (8.3 vs. 4.4 months; HR, 0.35). The greatest difference occurred in the cohort showing MET amplification, as PFS was 21.2 vs. 4.2 months here (HR, 0.17). Also, there was considerable benefit with respect to ORR in patients with high MET expression (68.4 % vs. 33.3 %) and MET amplification (66.7 % vs. 42.9 %). In the overall cohort, 45.2 % vs. 33.3 % of patients responded. Treatment with the combination of tepotinib and gefitinib was generally well tolerated, with most AEs showing mild or moderate intensity.
REFERENCES
- Camidge DR et al., Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK+ NSCLC. J Clin Oncol 36, 2018 (suppl; abstr 9043)
- Hida T et al., Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017; 390(10089): 29-39
- Zhou C et al., Primary results of ALESIA: a randomised, phase III, open-label study of alectinib versus crizotinib in Asian patients with treatment-naïve ALK+ advanced NSCLC. ESMO 2018, abstract LBA10
- Camidge DR et al., Brigatinib versus crizotinib in ALK-positive non-small cell lung cancer. N Engl J Med 2018
- Popat S et al., Intracranial efficacy of brigatinib vs crizotinib in the phase 3 ALTA-1L trial. ESMO 2018, abstract LBA58
- Felip E et al., Phase II study of ceritinib in ALK inhibitor (ALKi)-naïve patients with ALK-rearranged non-small cell lung cancer: final efficacy and safety results from ASCEND-3. ESMO 2018, abstract LBA57
- Felip E et al., Phase 2 study of ceritinib in ALKi-naïve patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC): whole body responses in the overall pt group and in pts with baseline brain metastases (BM). Ann Oncol 2016; 27(suppl 6): 416-454
- Cho B et al., ASCEND-8: A Randomized Phase 1 Study of Ceritinib, 450 mg or 600 mg, Taken with a Low-Fat Meal versus 750 mg in Fasted State in Patients with Anaplastic Lymphoma Kinase (ALK)-Rearranged Metastatic Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol 2017; 12(9): 1357-1367
- Cho B et al., Primary efficacy and updated safety of ceritinib (450 mg or 600 mg) with food vs. 750 mg fasted in ALK+ metastatic NSCLC (ASCEND-8). ESMO 2018, abstract LBA59
- Reungwetwattana T et al., The race to target MET exon 14 skipping alterations in non-small cell lung cancer: The Why, the How, the Who, the Unknown, and the Inevitable. Lung Cancer 2017; 103: 27-37
- Gelsomino F et al., MET and small-cell lung cancer. Cancers (Basel) 2014; 6(4): 2100-2115
- Ma PC, MET receptor juxtamembrane exon 14 alternative spliced variant: novel cancer genomic predictive biomarker. Cancer Discov 2015; 5(8): 802-805
- Awad MM et al., Impact of MET inhibitors on survival among patients (pts) with MET exon 14 mutant (METdel14) non-small cell lung cancer (NSCLC). J Clin Oncol 35, 2017 (suppl; abstr 8511)
- Sabari JK et al., PD-L1 expression and response to immunotherapy in patients with MET exon 14-altered non-small cell lung cancers (NSCLC). J Clin Oncol 35, 2017 (suppl; abstr 8512)
- Sabari JK et al., PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol 2018; 29(10): 2085-2091
- Fujino T et al., In vitro evaluation for optimal MET-TKI selection in lung cancers with MET mutations including exon 14 skipping WCLC 2018, abstract P1.13-41
- Wolf J et al., GEOMETRY Mono-1: phase II, multicenter study of MET inhibitor capmatinib (INC280) in EGFR wt, MET-dysregulated advanced NSCLC. WCLC 2017, abstract P2.04-005
- Shih K et al., SNO 2016, Poster ACTR-45
- Wolf J et al., Results of the GEOMETRY mono-1 phase II for evaluation of the MET inhibitor capmatinib (INC280) in patients with METΔEx14 mutated advanced non-small cell lung cancer. ESMO 2018, abstract LBA52
- Baldacci S et al., Outcome of EGFR-mutated NSCLC patients with MET-driven resistance to EGFR tyrosine kinase inhibitors. Oncotarget 2017; 8(62): 1051030-105114
- Friese-Hamim M et al., The selective c-Met inhibitor tepotinib can overcome epidermal growth factor receptor inhibitor resistance mediated by aberrant c-Met activation in NSCLC models. Am J Cancer Res 2017; 7(4): 962-972
- Cheng Y et al., Phase 2 study of tepotinib + gefitinib in MET-positive/epidermal growth factor receptor-mutant NSCLC. ESMO 2018, abstract 13770
- Wu YL et al., Phase Ib study of tepotinib in EGFR-mutant/c-Met-positive NSCLC: Final data and long-term responders. J Clin Oncol 35, 2017 (suppl; abstr 8547)