J-AXEL: nab-paclitaxel at least equal to docetaxel in pretreated NSCLC
Various advantages have been described for nab-paclitaxel, the albumin-bound, solvent-free, nanoparticle formulation of paclitaxel [1-3]. Phase II data showed favorable results in patients with pretreated advanced NSCLC who obtained an ORR of 32 % and median PFS of 5 months [4]. Therefore, the randomized, phase III study reported by Nakamura et al. compared nab-paclitaxel 100 mg/m2 on days 1, 8 and 15 three-weekly with docetaxel 60 mg/m2 every three weeks in patients with stage IIIB/IV or recurrent NSCLC previously treated with cytotoxic chemotherapy [5]. The analysis aimed to demonstrate non-inferiority of nab-paclitaxel with respect to OS. Both arms included approximately 250 patients.
Significant PFS and ORR benefits
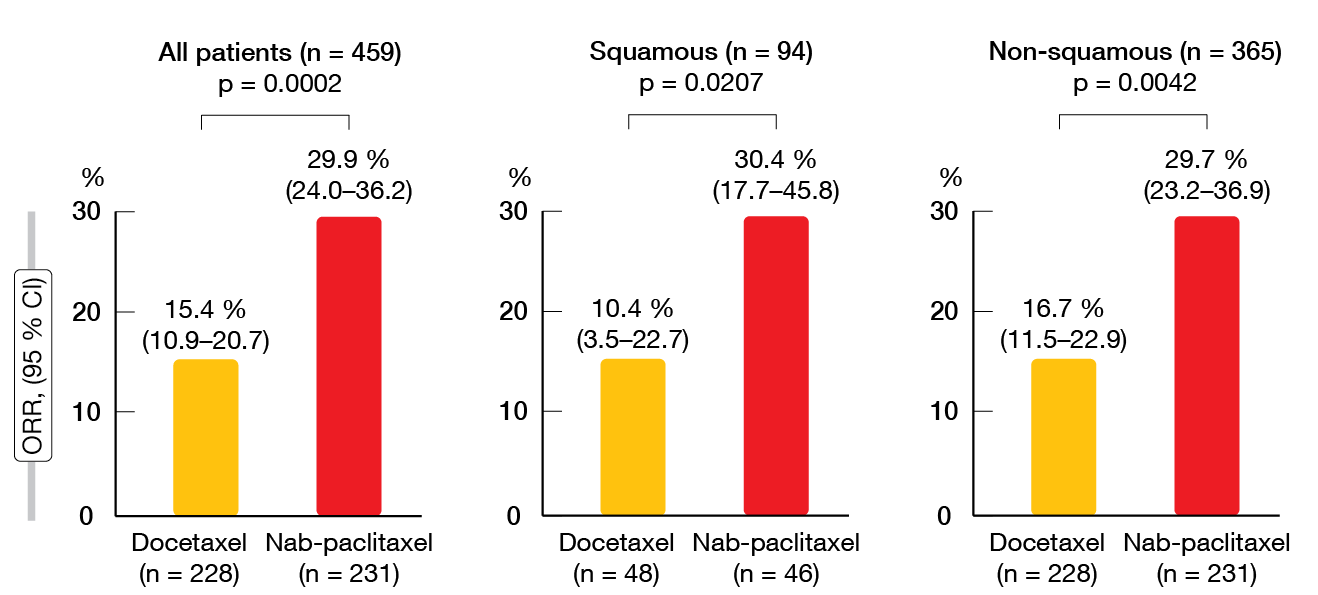
Non-inferiority of nab-paclitaxel in terms of OS was confirmed with the protocol-specified margin of 1.25 in the intent-to-treat population (HR, 0.85; 95.2 % CI, 0.68–1.070). Median OS amounted to 16.2 and 13.6 months with nab-paclitaxel and docetaxel, respectively. As specified by the protocol, superiority of nab-paclitaxel over docetaxel for OS was tested after non-inferiority had been shown. However, nab-paclitaxel did not significantly improve survival, although this was the case for both PFS and ORR. Median PFS was 4.2 vs. 3.4 months with nab-paclitaxel and docetaxel (HR, 0.76; p = 0.0042). In the total group (n = 459), 29.9 % vs. 15.4 % of patients responded to treatment (p = 0.0002; Figure). For the patients with squamous histology (n = 94), this was 30.4 % vs. 10.4 % (p = 0.0207), and for those with non-squamous NSCLC (n = 365), 29.7 % vs. 16.7 % (p = 0.0042). The results for both PFS and OS favored nab-paclitaxel across various subgroups pertaining to age, sex, ECOG performance status, histology, smoking status, disease stage, EGFR mutation status, and pretreatment.
Hematologic toxicity with docetaxel and neuropathy with nab-paclitaxel
Among AEs, leukopenia and neutropenia occurred significantly more often with docetaxel than with nab-paclitaxel (p < 0.0001 for both comparisons); correspondingly, docetaxel conferred a significantly higher incidence of febrile neutropenia (22.1 % vs 2.0 %). On the other hand, peripheral sensory neuropathy was more frequent with nab-paclitaxel (55.5 % vs. 20.1 %, p < 0.0001). The authors stressed in their summary that nab-paclitaxel should be considered a standard option for previously treated patients with advanced NSCLC.
Figure: Objective responses rates achieved with nab-paclitaxel vs. docetaxel in the ITT population and according to histology
REFERENCES
- Desai N et al., Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006; 12(4): 1317-1324
- Sasaki Y et al., Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci 2014; 105(7): 812-817
- Gradishar WJ et al., Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 2009; 27(22): 3611-3619
- Sakata S et al., Phase II trial of weekly nab-paclitaxel for previously treated advanced non-small cell lung cancer: Kumamoto thoracic oncology study group (KTOSG) trial 1301. Lung Cancer 2016; 99: 41-45
- Nakamura A et al., Phase III study comparing nab-paclitaxel with docetaxel in patients with previously treated advanced non-small cell lung cancer_J-AXEL. WCLC 2020, OA03.05
© 2020 Springer-Verlag GmbH, Impressum
More posts
KRAS, HER2 & ALK: targeted options and sequencing issues
KRAS, HER2 & ALK: targeted options and sequencing issues Deep responses wit
Pushing the bounds in early-stage lung cancer
Pushing the bounds in early-stage lung cancer ADAURA: role of adjuvant chemothe
Combinational immunotherapy of nivolumab and ipilimumab prolongs survival in comparison to standard chemotherapy in malignant pleural mesothelioma
Combinational immunotherapy of nivolumab and ipilimumab prolongs survival in compar
Preface
Preface Ross A. Soo, MB BS, PhD, FRACP, Department of Haematology-Oncology, Nati