Pushing the bounds in early-stage lung cancer
ADAURA: role of adjuvant chemotherapy
Approximately 30 % of patients with non-small-cell lung cancer (NSCLC) present with resectable disease at diagnosis [1-3]. Surgery with curative intent is the recommended treatment here, followed by adjuvant cisplatin-based chemotherapy in stage II/IIIA and select cases of stage IB disease [4-6]. However, recurrence rates remain high across disease stages, regardless of postoperative chemotherapy use [7]. The randomized, double-blind, phase III ADAURA study has revealed a highly statistically significant and clinically meaningful improvement in disease-free survival (DFS) with the adjuvant administration of the third-generation EGFR tyrosine kinase inhibitor (TKI) osimertinib (HR, 0.20; p < 0.0001) in patients with completely resected stage IB-IIIA, EGFR-mutated NSCLC [8, 9]. Osimertinib (n = 339) was compared to placebo (n = 343) with and without concomitant use of adjuvant chemotherapy. In both treatment arms, 60 % of patients received chemotherapy for a median of 4 cycles prior to randomization. At WCLC 2020, Wu et al. reported an exploratory analysis relating to adjuvant chemotherapy use and outcomes observed in ADAURA [10].
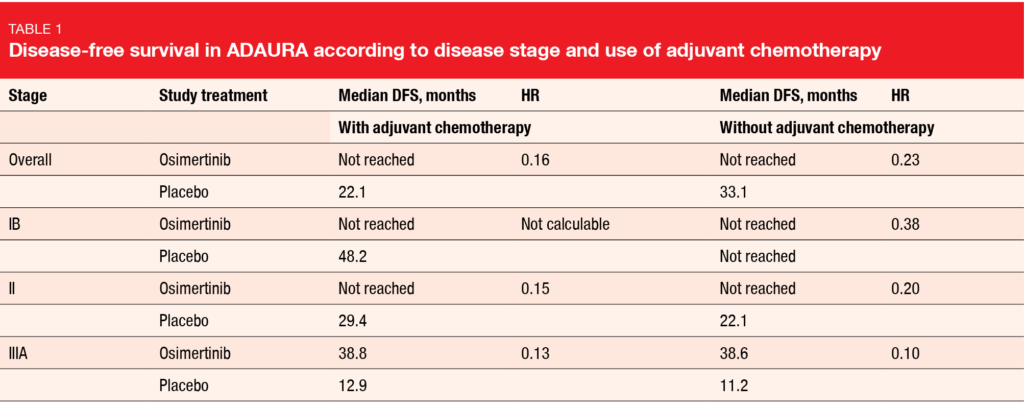
As expected, higher-stage disease and younger age (< 70 years) were generally associated with increased adjuvant chemotherapy use as compared to lower disease stage and older age, while WHO performance status (0 or 1) did not affect the treatment decision. Overall, chemotherapy use was in keeping with observations from previous studies and clinical practice [11, 12]. DFS benefits obtained with osimertinib vs. placebo did not depend on whether chemotherapy had been administered or not. Patients after adjuvant chemotherapy achieved an 84 % risk reduction (median DFS, not reached vs. 22.1 months; HR, 0.16; Table 1); 24-month DFS rates were 89 % vs. 49 %. For those without adjuvant chemotherapy, the risk reduction amounted to 77 % (not reached vs. 33.1 months; HR, 0.23), with 24-month DFS rates of 89 % vs. 58 %.
Osimertinib consistently improved DFS across disease stages (Table 1). In the subgroup of patients with stage IB disease who received chemotherapy, the hazard ratio was not calculable due to the small sample size and low number of events. Higher recurrence rates observed among placebo-treated patients who received adjuvant chemotherapy compared with those who did not were likely driven by the large proportion of patients with stage II/IIIA disease, as disease stage is a prognostic factor for clinical outcome [4]. The authors concluded that these data support adjuvant osimertinib as a highly effective treatment for patients with stage IB/II/IIIA EGFR-mutant NSCLC after resection with or without adjuvant chemotherapy.
Patient-reported outcomes from ADAURA
An important goal of adjuvant treatment is to improve efficacy outcomes while also maintaining health-related quality of life (HRQoL). Limited HRQoL data are available in the adjuvant NSCLC setting to date. ADAURA is the first global, randomized, phase III trial in patients with resected EGFR-mutant NSCLC to evaluate HRQoL outcomes with adjuvant EGFR TKI treatment compared to placebo, with or without prior adjuvant chemotherapy [13-17]. HRQoL was measured using the health survey SF-36 until disease recurrence, treatment completion at 3 years or treatment discontinuation, whichever came first. At data cutoff, median duration of total exposure was 22.5 months for osimertinib and 18.7 months for placebo.
The findings presented at WCLC 2020 showed that HRQoL was not affected by osimertinib treatment with and without chemotherapy in completely resected and disease-free patients [18]. No clinically meaningful differences between osimertinib and placebo were observed from baseline to week 96 for physical and mental component summary T-scores or health domain T-scores (i.e., physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, mental health).
During the disease-free period, > 80 % of patients across both arms did not experience any clinically meaningful deterioration in physical or mental component summary scores. For those who had deterioration, there were no differences in time to deterioration for any of the two summary scores between osimertinib and placebo. Moreover, time to deterioration of the SF-36 health domains did not differ across the treatment groups. Overall, despite prolonged treatment in the experimental arm of ADAURA, HRQoL was maintained, which further corroborates the significance of osimertinib as a new treatment strategy in this setting.
Interim findings for icotinib
The ongoing randomized, open-label, phase III EVIDENCE study is assessing the first-generation EGFR TKI icotinib, which has been approved for first-line monotherapy of EGFR-mutated NSCLC in China, in the adjuvant setting. Patients with completely resected, stage II-IIIA NSCLC were randomized to either icotinib 125 mg 3 times daily for 2 years (n = 161) or chemotherapy with cisplatin plus vinorelbine or pemetrexed depending on histology for 4 cycles (n = 161). DFS constitutes the primary endpoint. Approximately two thirds and one third of patients in each arm belong to the tumor stage categories IIA and IIIA, respectively, while only a minority has IIB disease. Lobectomy was performed in approximately 90 %.
As the interim analysis reported at WCLC 2020 showed, adjuvant icotinib significantly prolonged DFS compared to chemotherapy, with a risk reduction of 64 % (46.95 vs. 22.11 months; HR, 0.36; p < 0.0001) [19]. OS results were not mature yet. After a median duration of treatment of 22.2 months and 2.8 months with icotinib and chemotherapy, respectively, the safety analysis yielded no new signals. The most common grade 3/4 treatment-related adverse events (AEs) in the icotinib arm were rash (1.9 %), diarrhea (0.6 %) and dry skin (0.6 %). In the chemotherapy arm, these were neutropenia (41.0 %), leucopenia (19.4 %), vomiting (12.9 %) and nausea (7.2 %). No cases of interstitial lung disease occurred in either group. In their summary, the authors emphasized that adjuvant icotinib might provide a new treatment option for patients with EGFR-mutant, early-stage NSCLC who have undergone complete tumor resection.
ITACA: chemotherapy customization
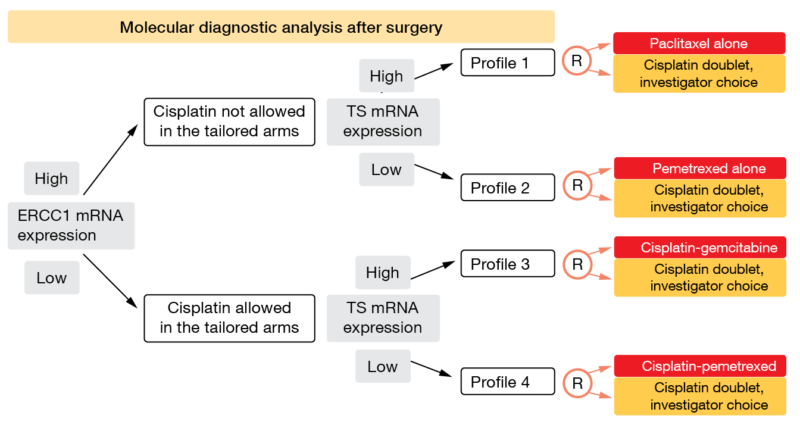
As the OS benefit of adjuvant platinum-based chemotherapy in early-stage NSCLC is modest, there is a clear need to better define patients most likely to derive survival improvement from this treatment while sparing those who do not need adjuvant chemotherapy. Based on the observation that mRNA expression of different genes has been correlated with the sensitivity or resistance to specific anticancer agents [20, 21], the phase III adjuvant ITACA trial aimed to evaluate the predictive utility of the mRNA expression levels of the molecular markers excision repair cross complementation 1 (ERCC1) and thymidylate synthase (TS) [22]. Randomization was performed following centralized assessment of ERCC1 and TS levels by real-time PCR on samples from completely resected, stage II-IIIA NSCLC. The first allocation pertained to high vs. low ERCC1 mRNA expression, which was followed by allocation to high vs. low TS mRNA expression in each of the ERCC1 groups (Figure 1).
This resulted in four groups characterized by distinct genomic profiles within which the patients were randomized to treatment. All patients in the four control arms received standard chemotherapy with cisplatin doublets according to investigator’s choice. In the ERCC1-high part of the population, cisplatin was not allowed in the tailored arms. TS mRNA expression was relevant for the decision between paclitaxel and pemetrexed in the tailored ERCC1-high groups and gemcitabine vs. pemetrexed in the tailored ERCC1-low groups. A total of 31 centers in Italy, Germany and Poland participated in the trial.
Figure 1: Design of the ITACA trial: randomization in four patient groups that have been allocated using ERCC1 and TS mRNA expression
No significant results in an underpowered sample
Between 2008 and 2014, 773 patients were randomized. For statistical purposes, all pharmacogenomic-driven arms were grouped together as the tailored arm (n = 344), and all control arms were grouped together as the standard arm (n = 346). OS was defined as the primary endpoint. In both arms, patients received a median of four cycles.
Adjuvant chemotherapy customization based on the primary tumor tissue mRNA expression of ERCC1 and TS did not result in significant OS improvement. A trend favoring the tailored approach was observed in the ITT population (96.4 vs. 83.5 months; HR, 0.76). At the time of the final analysis, the study was underpowered; only 46 % of expected events had occurred. No heterogeneity existed regarding OS between different genomic profiles, although the statistical power was very low. Likewise, recurrence-free survival did not differ significantly across the arms (64.4 vs. 41.5 months; HR, 0.94).
At the same time, treatment customization significantly improved the toxicity profile of treatment without compromising efficacy; this difference mainly related to hematological AEs. The odds ratio of ≥ 1 grade 3-4 event was 0.57 for the comparison between the tailored and control arms (p < 0.001). Considering the insufficient statistical power of the analysis, the authors concluded that more comprehensive and high-throughput diagnostic techniques will be needed to tailor adjuvant chemotherapy with or without immunotherapy in completely resected NSCLC.
EGFR TKI therapy for stage III EGFR-mutant disease
In the setting of unresectable, locally advanced NSCLC harboring EGFR mutations, the efficacy of early use of EGFR TKIs is unclear. A retrospective analysis investigated first-line treatment patterns in 440 patients with unresectable stage IIIA/IIIB NSCLC treated at 12 Chinese academic cancer institutions [23]. Group 1 received concurrent or sequential chemoradiation; group 2 underwent radiotherapy plus EGFR TKIs with or without chemotherapy; and group 3 had upfront TKI treatment alone until tumor progression. For their analysis, the researchers used inverse-probability of treatment weighting (IPTW) based on a multinomial propensity score model to reduce the effects of potential confounding factors while maximizing the effective sample sizes.
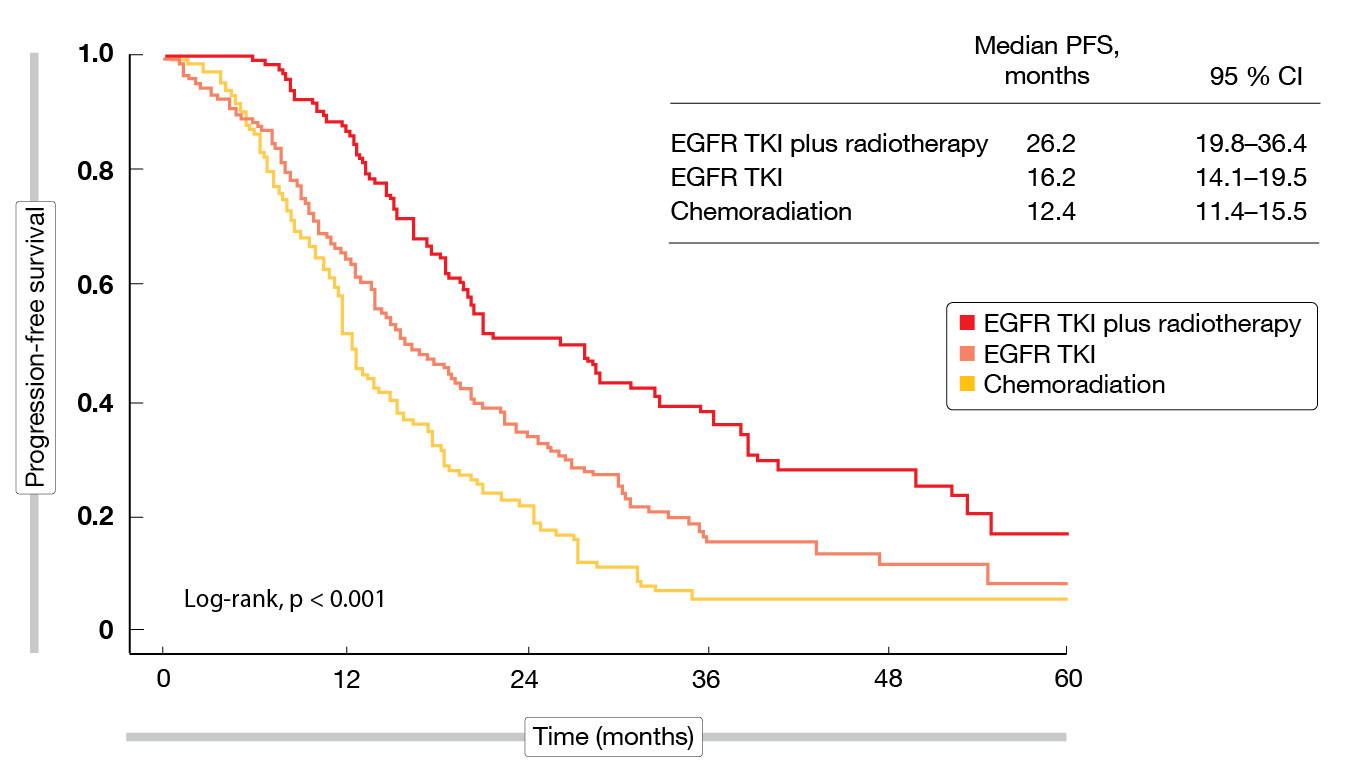
According to the IPTW analysis, TKI treatment plus radiotherapy with or without chemotherapy proved superior to both the standard chemoradiation approach and the TKI-only approach with respect to overall survival (OS) and progression-free survival (PFS). Median OS was 67.4 months with TKI plus radiotherapy vs. 51.0 months with chemoradiation (HR, 0.61; p = 0.039) and 49.3 months with TKI monotherapy. For PFS, this was 26.2 months vs. 12.4 months (HR, 0.40; p < 0.001) and 16.2 months (Figure 2). The OS benefit might be explained by the effective control of both local-regional and distant disease. Concerning locoregional failure, patients treated with TKI plus radiotherapy had the lowest risk compared to the other groups, with a 52 % risk reduction versus chemoradiation (HR, 0.48; p = 0.002). For distant progression, both patients receiving TKI plus radiotherapy and TKI monotherapy fared better than those in the chemoradiation group (HRs, 0.62 and 0.56, respectively; p = 0.013 and < 0.001, respectively).
The authors noted in their conclusion that the combination of treatment modalities will provide health benefits for a greater number of patients with unresectable, locally advanced NSCLC. Randomized, controlled trials with the aim of exploring irradiation plus EGFR TKI treatment with or without chemotherapy are warranted.
Figure 2: Progression-free survival for chemoradiation, EGFR TKI therapy and EGFR TKI plus radiotherapy after IPTW analysis
LCMC3: neoadjuvant atezolizumab
The neoadjuvant use of atezolizumab was investigated by the LCMC3 trial that included untreated patients with resectable NSCLC (i.e., unselected stage IB-IIIA and select stage IIIB). Prior to surgery, two cycles of atezolizumab were administered. Thereafter, the protocol permitted optional adjuvant atezolizumab for 12 months or stage-appropriate therapy according to investigator’s choice. At WCLC 2020, Lee et al. presented the primary analysis of the trial [24]. Out of 181 patients who constituted the safety population, 159 underwent surgery. A total of 144 individuals made up the primary efficacy population.
The primary endpoint of major pathologic response (MPR; defined as ≤ 10 % viable tumor cells) was met, with a rate of 21 % in the primary efficacy population. Seven percent of these patients achieved pathological complete response. Downstaging following atezolizumab therapy resulted in 43 %, while 19 % of patients upstaged. Resection was performed within the narrow protocol-defined window of ± 10 days from the completion of atezolizumab therapy in 88 % of cases. Median time from the end of cycle 2 to surgery was 22 days. Most patient underwent lobectomy (79 %); R0 resection was achieved in as many as 92 %.
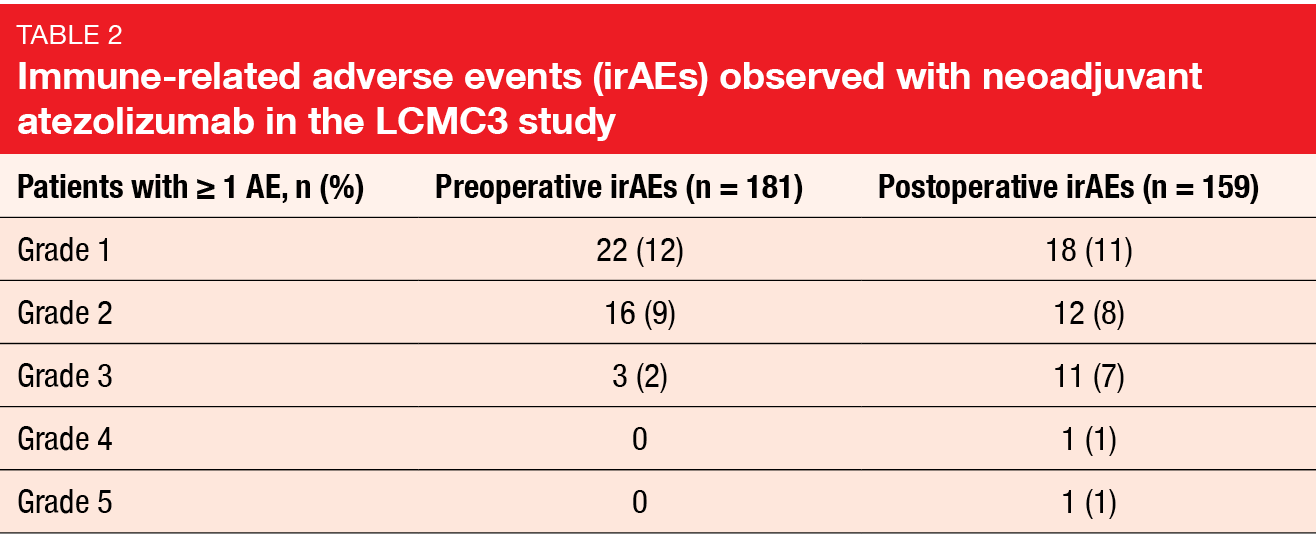
Perioperative morbidity and mortality were low. Intraoperative events occurred in 4 %, and all complications were successfully treated. One patient died within 30 after surgery due to sudden death, and another within the time window of 30–90 days after surgery due to pneumonitis. The median length of hospitalization was 7.5 days. Pre- and postoperative immune-related AEs were mostly grade 1 and 2 (Table 2).
Exploratory endpoints included efficacy outcomes in the primary efficacy population. DFS rates at 1.5 years were 79 % and 77 % for patients with stage I/II and stage III disease, respectively, while OS rates amounted to 91 % and 87 %, respectively. According to an analysis of clinical and biomarker data from LCMC3, patients who achieved MPR showed a trend towards OS and DFS improvement [25]. Also, better pathological responses were associated with STK11 wildtype, higher tumor mutational burden and a greater amount of activated immune cells and CD68-positive cells in the tumor microenvironment at baseline.
Overall, LCMC3 provides additional evidence for the ongoing placebo-controlled phase III IMpower030 study evaluating atezolizumab plus platinum-based chemotherapy.
Update of KEYNOTE-799
The standard of care for patients with stage III unresectable NSCLC includes concurrent chemoradiation (cCRT) and durvalumab as consolidation therapy in patients who have not progressed after ≥ 2 cycles of cCRT [26]. However, up to one third of patients might not be eligible for consolidation therapy with durvalumab [27, 28]. Therefore, the non-randomized phase II KEYNOTE-799 study investigated pembrolizumab plus cCRT in patients with stage IIIA-C, unresectable, previously untreated NSCLC. Cohort A that consisted of patients with both squamous and non-squamous NSCLC received pembrolizumab plus paclitaxel/carboplatin alone (cycle 1) and together with thoracic radiotherapy (cycles 2-3), which was followed by pembrolizumab monotherapy (cycles 4-17). Cohort B was restricted to patients with non-squamous NSCLC. Here, pembrolizumab plus pemetrexed/cisplatin was administered alone (cycle 1) and together with radiotherapy (cycle 2-3). In cycle 4-17, the patients received pembrolizumab monotherapy. At the time of the primary analysis, ORR was 67.0 % in cohort A and 56.6 % in cohort B [29]. Reck et al. reported the results of the study after an additional follow-up of 6 months [30].
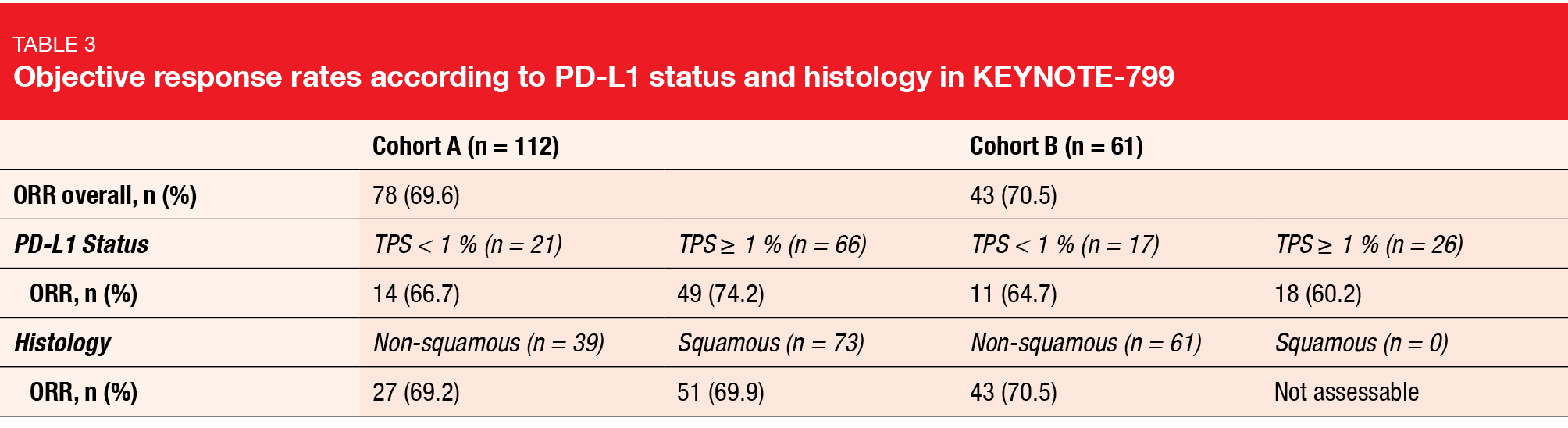
Pembrolizumab plus cCRT continued to show promising antitumor activity. In cohort A (n = 112), ORR was 69.6 %, and in cohort B (n = 61), 70.5 %. Similar percentages were observed across the subgroups determined by PD-L1 tumor proportion score (< 1 % vs. ≥ 1 %) and histology (non-squamous vs. squamous; Table 3). Median duration of response had not been reached yet in either cohort. In 82.2 % and 72.1 % in cohorts A and B, respectively, responses lasted for ≥ 12 months. Likewise, median PFS had not been reached in both arms, with 12-month PFS rates of 67.7 % and 65.2 %, respectively. Twelve-month OS rates amounted to 81.2 % and 88.0 %. Median OS was immature.
The incidence of AEs among patients who received pembrolizumab plus cCRT was consistent with the established toxicity profiles of cCRT for stage III NSCLC and pembrolizumab monotherapy [31, 32]. Grade ≥3 pneumonitis occurred in 8.0 % and 7.9 %, respectively, and was thus within the expected range for immunotherapy combined with cCRT [33].
REFERENCES
- Datta D, Lahiri B, Preoperative evaluation of patients undergoing lung resection surgery. Chest 2003; 123(6): 2096-2103
- Le Chevalier T, Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann Oncol 2010; 21 Suppl 7: vii196-198
- Cagle PT et al., Lung cancer biomarkers: present status and future developments. Arch Pathol Lab Med 2013; 137(9): 1191-1198
- Chansky K et al., The IASLC Lung Cancer Staging Project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2017; 12(7): 1109-1121
- Kris MG et al., Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice guideline update. J Clin Oncol 2017; 35(25): 2960–2974
- Postmus PE et al., Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28 (suppl4): iv1-iv21
- Pignon JP et al., Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008; 26(21): 3552–3559
- Herbst RS et al., Osimertinib as adjuvant therapy in patients with stage IB–IIIA EGFR mutation positive NSCLC after complete tumor resection: ADAURA. J Clin Oncol 38: 2020 (suppl; abstr LBA5)
- Wu YL et al., Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020; 383(18): 1711-1723
- Wu YL et al., Postoperative chemotherapy use and outcomes from ADAURA: osimertinib as adjuvant therapy for resected EGFR mutated NSCLC. WCLCL 2020, OA06.04
- Chouaid C et al., Adjuvant treatment patterns and outcomes in patients with stage IB-IIIA non-small cell lung cancer in France, Germany, and the United Kingdom based on the LuCaBIS burden of illness study. Lung Cancer 2018; 124: 310-316
- Buck PO et al., Treatment patterns and health resource utilization among patients diagnosed with early stage resected non-small cell lung cancer at US community oncology practices. Clin Lung Cancer 2015; 16(6): 486-495
- Zhong WZ et al., Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018; 19(1): 139-148
- Goss GD et al., Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013; 31(27): 3320-3326
- Kelly K et al., Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol 2015; 33(34): 4007-4014
- Clinicaltrials.gov NCT02193282
- Clinicaltrials.gov NCT02511106
- Majem M et al., Patient-reported outcomes from ADAURA: osimertinib as adjuvant therapy in patients with resected EGFR mutated (EGFRm) NSCLC. WCLC 2020, OA06.03
- Zhou C et al., Icotinib versus chemotherapy as adjuvant treatment for stage II-IIIA EGFR-mutant NSCLC (EVIDENCE): a randomized, open-label, phase 3 study. WCLC 2020, FP14.11
- Ceppi P et al., Thymidylate synthase expression in gastroenteropancreatic and pulmonary neuroendocrine tumors. Clin Cancer Res 2008; 14(4): 1059-1064
- Joshi MB et al., High gene expression of TS1, GSTP1, and ERCC1 are risk factors for survival in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res 2005; 11(6): 2215-2221
- Novello S et al., International tailored chemotherapy adjuvant (ITACA) phase III study of pharmacogenomic-driven versus standard adjuvant chemotherapy in completely resected stage II-IIIA non-small cell lung cancer. WCLC 2020, PS01.04
- Bi N et al., Results of inverse-probability of treatment weighting (IPTW) using propensity score from REFRACT: a multi-center study investigating the treatment patterns in EGFR-mutant unresectable LA-NSCLC. WCLC 2020, OA02.06
- Lee JM et al., Surgical and clinical outcomes with neoadjuvant atezolizumab in resectable stage IB-IIIB NSCLC: LCMC3 trial primary analysis. WCLC 2020, PS01.05
- Carbone D et al., Clinical/biomarker data for neoadjuvant atezolizumab in resectable stage IB-IIIB NSCLC: primary analysis in the LCMC3 study. WCLC 2020, OA06.06
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 1.2021). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. 2.
- Agulnik J et al., Understanding clinical practice and survival outcomes in patients with unresectable stage III non-small-cell lung cancer in a single centre in Quebec. Curr Oncol 2020; 27(5): e459-e466
- Horinouchi H et al., Real-world outcomes of chemoradiotherapy for unresectable Stage III non-small cell lung cancer: The SOLUTION study. Cancer Med 2020; 9(18): 6597-6608
- Jabbour SK et al., Phase II study of pembrolizumab (pembro) plus platinum doublet chemotherapy and radiotherapy as first-line therapy for unresectable, locally advanced stage III NSCLC: KEYNOTE-799. J Clin Oncol 2020; 38(15 suppl): 9008
- Reck M et al., Pembrolizumab plus platinum chemotherapy and radiotherapy in unresectable, locally advanced, stage III NSCLC: KEYNOTE-799. WCLC 2020, OA02.03
- Yoon SM et al., Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol 2017; 8(1): 1-20
- Mok TS et al., Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393(10183): 1819-1830
- Peters S et al., Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-the ETOP NICOLAS trial. Lung Cancer 2019;133: 83-87
© 2020 Springer-Verlag GmbH, Impressum