Specific treatment approaches in the EGFR-mutated setting
Exon 20 insertions: phase I/II data for mobocertinib
EGFR exon 20 insertion mutations are found in approximately 5 % to 12 % of EGFR-mutated NSCLC tumors, i.e., in 2 % of all NSCLC cases [1, 2]. They represent the third most common EGFR mutation after L858R and exon 19 deletion [1, 3]. However, EGFR TKIs cannot be used to treat lung cancer with exon 20 insertions as they are insensitive to these drugs due to steric hindrance at the TKI-binding site [4]. No specific targeted therapies have been approved for the treatment of these patients to date. First- and second-generation EGFR TKIs or chemotherapy provide objective response rates of approximately 10 % to 15 % and median PFS of 3 to 5 months [5-10].
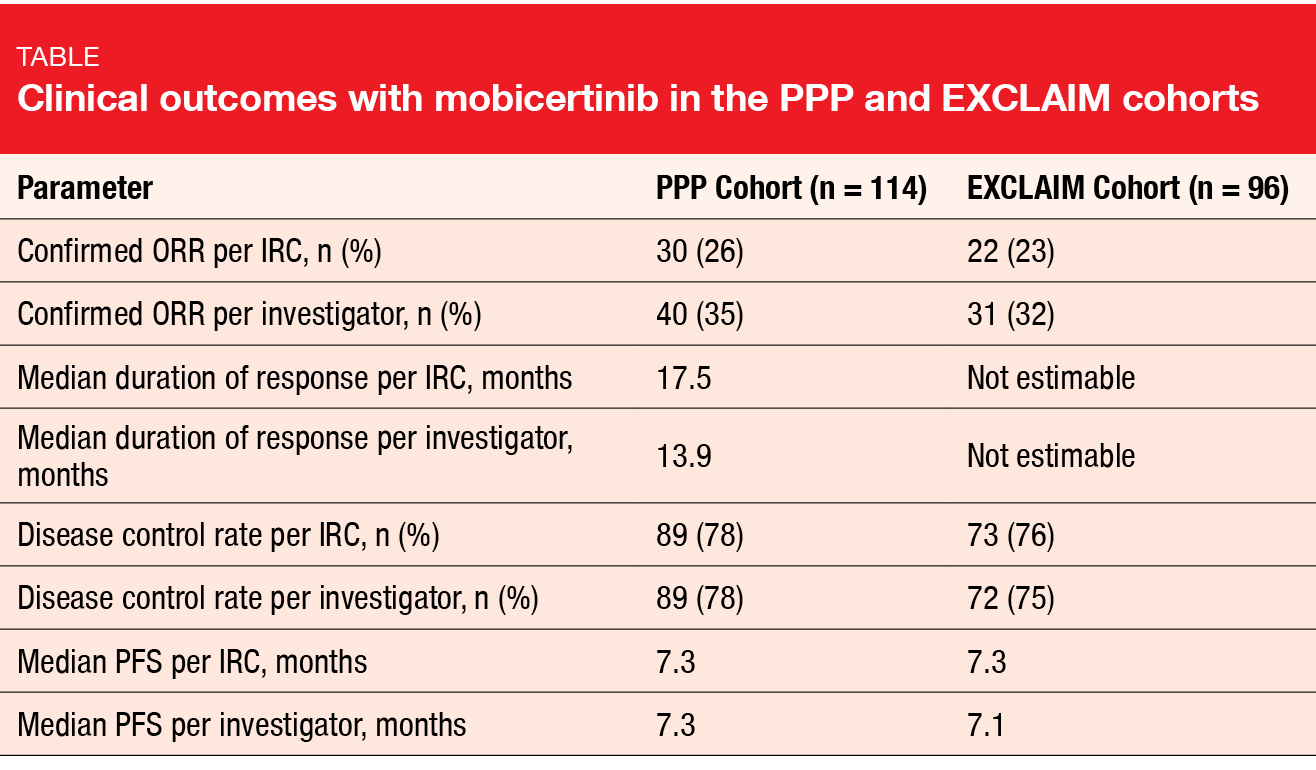
Mobocertinib (TAK-788) is a first-in-class, potent, oral TKI targeting EGFR exon 20 in-frame insertion mutations. Based on preliminary results from a phase I/II study, mobocertinib was granted a Breakthrough Therapy Designation in the USA and China for the treatment of NSCLC patients with exon 20 insertion in whom chemotherapy has failed [11]. At WCLC 2020, Zhou et al. reported findings obtained with mobocertinib in patients with metastatic NSCLC and EGFR exon 20 insertion mutations from the platinum-pretreated group (PPP cohort) included in the phase I/II study and the extension cohort (EXCLAIM) [12]. The PPP and EXCLAIM cohorts comprised 114 and 96 patients, respectively.
Long-lasting improvements
In both groups, treatment with mobocertinib demonstrated meaningful benefits. ORR per independent review committee (IRC) was 26 % in the PPP cohort, and responses lasted for a median of 17.5 months (Table). Median PFS was 7.3 months. For the EXCLAIM cohort, ORR and median PFS were 23 % and 7.3 months, respectively, while median duration of response had not been reached yet. Although numerical differences were seen between the IRC and investigator assessments in both groups, similar disease control rates and PFS suggested that the magnitude of clinical benefit was the same with both assessments.
Duration of response > 6 months was observed in 78 % and 84 % of patients in the PPP and EXCLAIM cohorts, respectively. At the time of data cutoff, over 50 % of responses were ongoing in the entire population. Reductions in the sum of target lesion diameter from baseline resulted in 82 % and 80 %, respectively. Confirmed responses with mobocertinib were similar among all prespecified subgroups (i. e., Asian vs. non-Asian, pretreatment with immunotherapy or EGFR TKI, presence of brain metastases at baseline).
The safety profile was consistent with the known profile of EGFR TKIs. Diarrhea and rash occurred as the most common treatment-related AEs. Grade 3/4 diarrhea was observed in 21 % and 16 % in the PPP and EXCLAIM cohorts, respectively. Nausea and diarrhea emerged as the most common AEs leading to treatment discontinuation. Overall, the treatment discontinuation rates due to AEs in the two cohorts amounted to 17 % and 10 %, respectively. Dose reductions became necessary in 25 % and 21 %, respectively. One treatment-related death occurred due to cardiac failure in a platinum-pretreated patient in the EXCLAIM cohort.
The analysis included an assessment of symptom scores. In the EXCLAIM cohort, mobocertinib gave rise to clinically meaningful improvements in core lung cancer symptoms (i.e., ≥ 10-point decrease in the EORTC QLQ-LC13 symptom score) from cycle 2 that were maintained throughout the treatment period. Meaningful changes were evident for dyspnea (54.4 % of patients), cough (44.4 %), and chest pain (37.8 %).
Robust efficacy of amivantamab
Another agent that has received Breakthrough Therapy Designation for exon 20 insertion-positive NSCLC in the USA and China is the bispecific antibody amivantamab that targets activating and resistance EGFR mutations as well as MET mutations and amplifications [13, 14]. The CHRYSALIS trial established the recommended phase II dose for antivantamab at 1,050 mg and 1,400 mg in patients with a body weight of < 80 kg and ≥ 80 kg, respectively. In the dose expansion part of the study, the safety and efficacy of this regimen was tested in patients with metastatic/unresectable NSCLC and EGFR exon 20 insertion mutations after progression on platinum-based chemotherapy. Sabari et al. presented the findings for the efficacy population who had undergone at least three disease assessments at clinical cutoff (n = 81) and the safety population treated with the recommended phase II dose (n = 114) [15]. The median number of prior treatment lines was 2 in the efficacy population. Twenty-five percent were EGFR-TKI–pretreated, and immunotherapy had been administered in 46 %.
Amivantamab showed robust efficacy with an ORR of 40 % according to blinded IRC. The clinical benefit rate (i. e., complete or partial response or stable disease for ≥ 2 assessments) was 74 %. Median duration of response amounted to 11.1 months. At the time of data cutoff, 47 % of patients remained on treatment. Median PFS and OS were 8.3 and 22.8 months, respectively. Antitumor activity of the treatment was observed in all subgroups and across different insertion regions of the EGFR exon 20 (i. e., helical region, near loop, far loop). Overall, the efficacy of avivantamab compared favorably to currently available treatment options for NSCLC patients with exon 20 insertion mutations [16].
Also, the bispecific antibody showed a tolerable safety profile that was consistent with the known profiles observed in the setting of EGFR and MET pathway inhibition. Rash and infusion-related reactions occurred as the most common TEAEs. However, only 2 % of patients discontinued treatment because of rash, and almost all of the infusion-related reactions were observed at the first administration and rarely impacted the ability to continue the therapy. Treatment-related grade ≥ 3 AEs emerged in 16 % and led to discontinuation in 4 %. Based on these results, the combined use of amivantamab with other drug classes is currently being evaluated.
PCR testing fails in 50 %
As new drugs are being developed for the treatment of patients with exon 20 insertion-positive NSCLC, reliable identification of exon 20 insertion mutations, which are molecularly heterogeneous, is gaining importance. The most commonly used testing methods are polymerase chain reaction (PCR) and next-generation sequencing (NGS). However, as Bauml et al. showed, many cases tend to go undetected with PCR testing [17]. The researchers assessed the ability of PCR and NGS tests to comprehensively identify EGFR exon 20 insertion variants in US patients with NSCLC. To this end, two real-world databases were analyzed, which were the AACR Project Genomics Evidence Neoplasia Information Exchange database and the FoundationInsights database.
Results from both databases demonstrated that PCR missed approximately half of patients with exon 20 insertions identified by NGS. There was a wide range of unique exon 20 variants according to NGS (40-102), which suggests that NGS platforms, academic or commercially available, would improve their detection rate by capturing the full breadth of variants.
HER3-directed ADC patritumab deruxtecan
Most lung cancers, including > 80 % of EGFR-mutated NSCLC, express HER3, which represents a promising therapeutic target. Overexpression of HER3 has been associated with worse clinical outcomes [18-20]. To date, no HER3-directed therapies have been approved. The novel, investigational HER3-directed antibody-drug conjugate patritumab deruxtecan has been designed to contain an anti-HER3 IgG1 monoclonal antibody covalently linked to a topoisomerase I inhibitor payload. It is being evaluated in a global, multicenter, open-label phase I study conducted in patients with EGFR-mutant, metastatic/unresectable NSCLC.
The dose escalation part of the trial included patients who were either progressive after osimertinib or T790M-negative after progression on erlotinib, gefitinib, or afatinib. Here, the recommended dose for expansion was determined at 5.6 mg/kg i. v. every 3 weeks. Patients in the dose expansion portion of the study were enrolled into 3 cohorts; data for those in cohort 1 were included in the analysis presented at WCLC 2020 by Yu et al. [21]. This group had previously been treated with ≥ 1 EGFR TKI and ≥ 1 platinum-based chemotherapy regimen.
As of April 30, 2020, 57 patients from both parts of the study had received 5.6 mg/kg of patritumab deruxtecan, with 56 being evaluable for response. The median number of prior therapies for advanced or metastatic disease was 4. Forty-seven percent of patients had a history of CNS metastases.
Early benefits irrespective of resistance aberrations
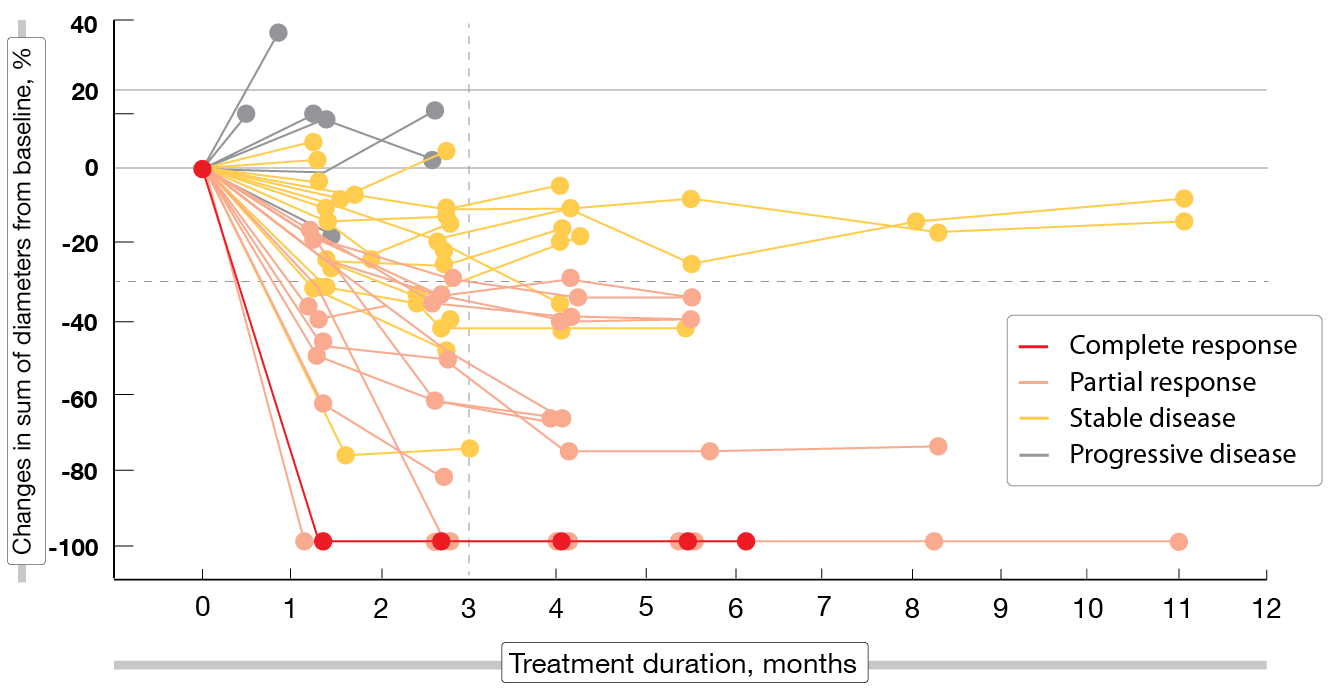
After a median follow-up of 5 months, patritumab deruxtecan 5.6 mg/kg demonstrated clinically meaningful antitumor activity in this heavily pretreated population with EGFR-mutated NSCLC and various TKI resistance mechanisms. These included EGFR C797S mutation, MET amplification, HER2 mutation, BRAF fusion, and PIK3CA mutation. Overall, 25 % of patients responded and 70 % achieved disease control, although 3 partial responses had not been confirmed yet and 6 patients had undergone only one tumor evaluation at the time of the analysis. One patient (2 %) obtained complete response. Decreases in tumor size occurred within 3 months (Figure 1). Median duration of response was 6.9 months.
Patritumab deruxtecan showed a manageable safety profile, with thrombocytopenia and neutropenia being the most common grade ≥ 3 TEAEs. In 9 %, TEAEs led to treatment discontinuation. Three interstitial lung disease events (5.3 %) were adjudicated by an IRC as being related to treatment. No grade 5 AEs occurred. In their entirety, these insights support further clinical investigation of patritumab deruxtecan in a patient population with no available targeted options, as the authors noted in their summary. The phase II HERTHENA-Lung01 study investigating single-agent patritumab deruxtecan is currently enrolling patients after failure of EGFR TKIs and platinum-based chemotherapy (NCT04619004).
Figure 1: Patritumab deruxtecan: changes in tumor size over time (n = 49)
Impressive activity of front-line mefatinib
The second-generation EGFR TKI mefatinib that binds irreversibly to mutated EGFR inhibits EGFR- and HER2-overexpressing, EGFR-mutant and KRAS-mutant lung cancer, as well as other HER2- and EGFR-overexpressing cancer types. A randomized, open-label phase II study involving 106 patients with EGFR-mutant stage IIIB/IV NSCLC was conducted to assess the efficacy and safety of first-line mefatinib 60 mg and 80 mg orally once daily [22]. The analysis yielded a substantial ORR of 84.9 % in the total population; for the 60 mg and 80 mg doses, this was 80.4 % and 89.1 %, respectively. Disease control resulted in 97.2 % overall. Median PFS and OS amounted to 16.3 and 26.6 months in the total population. Mefatinib was well tolerated. Any-grade AEs mainly included diarrhea (94.3 %) and rash (86.8 %). Among grade ≥ 3 events, the most common AEs were diarrhea (19.8 %), rash (17 %), mouth ulceration (4.7 %), and stomatitis (4.7 %).
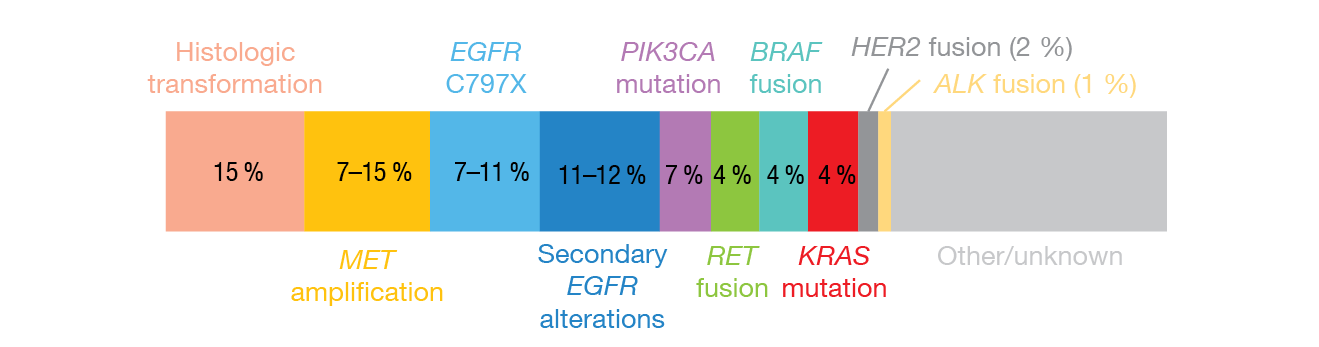
The trial included a biomarker study aimed at exploring predictive biomarkers and potential molecular mechanisms of acquired resistance to mefatinib. Circulating tumor DNA (ctDNA) clearance was defined as the absence of any mutation on a panel of 168 lung-cancer-related genes. Patients who experienced clearance of ctDNA at the first follow-up 6 weeks from starting mefatinib therapy had significantly longer PFS (p = 0.01; Figure 2) and OS (p = 0.005) than mutation-positive individuals. As of data cutoff, 38 patients experienced disease progression. Here, the most prevalent mechanism of acquired resistance to mefatinib was the EGFR T790M mutation (42.1 %). Three patients with EGFR T790M also acquired concurrent bypass resistance mechanisms, which were BRAFV600E mutation (n = 2) and MET amplification (n = 1). In 18 cases (48 %), no known resistance mechanism was detected.
Figure 3: The most common acquired resistance mechanisms identified in patients treated with first-line osimertinib
RET fusions: osimertinib plus selpercatinib
Acquired oncogenic RET fusions are present in approximately 5 % of patients developing resistance to first-line osimertinib [26]. Rotow et al. systematically characterized the clinical response to osimertinib plus the selective RET inhibitor selpercatinib in patients with EGFR-mutant NSCLC who had acquired RET fusions on osimertinib treatment [27]. Data for 11 patients were collected across all selpercatinib compassionate access programs.
In this group, osimertinib plus selpercatinib proved active, with an ORR of 50 % and a disease control rate of 80 %. Only one patient developed progressive disease. For responders, the median treatment duration was 11 months. The combination therapy was generally well tolerated. Adverse effects were consistent with the known profiles of the two drugs. One patient discontinued treatment due to grade 2 pneumonitis. Dose reductions for selpercatinib and osimertinib became necessary in one patient each. Grade 3 treatment-related AEs included hypertension, QTc prolongation, neutropenia and leukopenia. Formal AE reporting was voluntary with the exception of serious AEs.
The results for acquired resistance to osimertinib plus selpercatinib were heterogeneous and mirrored those seen in the setting of acquired resistance to TKI monotherapy. Aberrations included the second-site resistance mutations EGFR C797S and RET G810S. At present, osimertinib plus selpercatinib is prospectively assessed in the ORCHARD study in EGFR-positive NSCLC patients with acquired RET fusion [25].
Afatinib in EGFR G724S-positive disease
EGFR exon 18 G724S is a rare mutation mediating resistance to third-generation EGFR TKI treatment, although it retains sensitivity for second-generation EGFR TKIs including afatinib [28, 29]. Zhao et al. investigated afatinib in patients with G724S-positive NSCLC whose data were retrieved from a database comprising 42,316 individuals with lung cancer [30]. Twenty-three patients entered the survival analysis. In addition, an analysis of concurrent mutations in EGFR and other genes was conducted in 52 patients. This showed that 75 % harbored concurrent EGFR exon 19 deletions, with the rare variant E746_S752 delinsV being the most common one (55 %). Fifteen percent harbored concomitant exon 20 point mutations. The authors concluded that EGFR G724S, as a resistance mutation, emerges preferentially in the context of E746_S752delinsV, while G724S co-occurring with the EGFR exon 20 mutation is more likely a primary mutation.
All afatinib-treated patients with the G724S mutation (n = 8) achieved stable disease, which resulted in a disease control rate of 100 %. Compared to non-afatinib therapies (n = 15), PFS was significantly longer (4.5 vs. 1.7 months; HR, 0.33; p = 0.04). In the subset of patients who had progressed on osimertinib, afatinib (n = 5), compared to non-afatinib therapies (n = 8), also induced superior PFS (6.2 vs. 1.0 months; HR, 0.04; p = 0.006). According to the authors, afatinib monotherapy is a potential therapeutic option for NSCLC patients with the EGFR G724S mutation. The EGFR T790M mutation re-emerged as a major resistance mechanism to afatinib in two osimertinib-treated patients, while MET amplification mediated resistance in one patient treated with first-line afatinib.
First- and second-line EGFR TKIs vs. osimertinib
Compared to first-generation EGFR TKIs, the FLAURA study has shown PFS and OS benefits of osimertinib as first-line therapy in patients with EGFR-mutated, advanced NSCLC [31]. A US-based, multicenter, retrospective review assessed the outcomes of patients treated with first/second-generation EGFR TKIs or osimertinib in the front-line setting between 2014 and 2019 [32]. Overall, 172 patients were included; among these, 52 (30.2 %) had received osimertinib, while 120 (69.8 %) had been treated with either afatinib (n = 25; 14.5 %), gefitinib (n = 1; 0.6 %), or erlotinib (n = 94; 54.7 %).
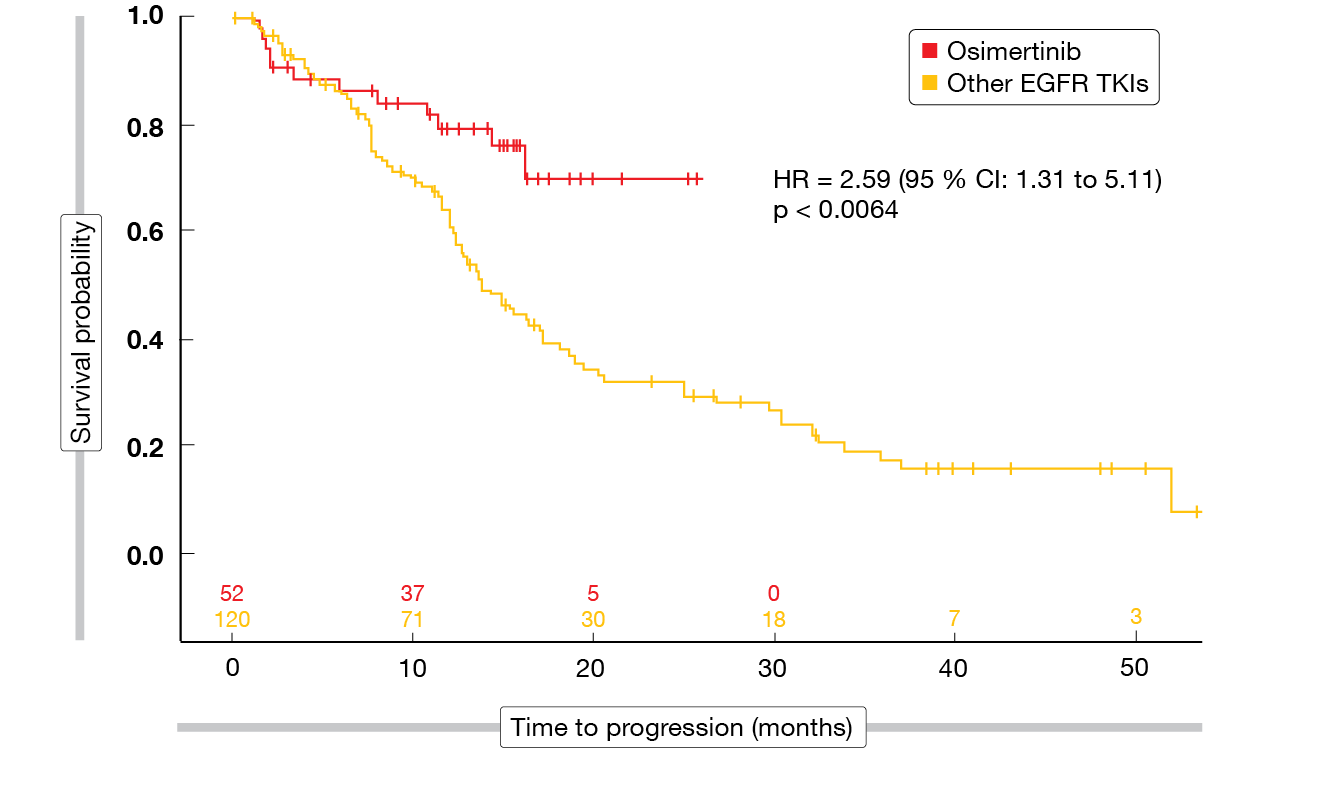
For the analysis, the population was dichotomized (osimertinib vs. all other EGFR TKIs). All of the baseline characteristics were comparable across the two groups with the exception of total bilirubin levels that were higher in the cohort receiving other EGFR TKIs. Osimertinib gave rise to improved PFS compared to the other EGFR TKIs at 12 and 18 months (HR, 2.59; p < 0.0064; Figure 4), while OS did not differ significantly between the groups (HR, 0.95; p < 0.9128). As the authors noted, additional studies and longer follow-up are needed to solidify the real-world OS benefit of osimertinib.
Figure 4: Real-world progression-free survival with osimertinib vs. other EGFR TKIs as first-line agents in EGFR-mutant NSCLC
REFERENCES
- Riess JW et al., Diverse EGFR exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol 2018; 13(10): 1560-1568
- Fang W et al., EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer 2019; 19(1): 595
- Vyse S, Huang PH, Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 2019; 4: 5
- Robichaux JP et al., Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018: 24(5): 638-646
- Yasuda H et al., Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013; 5(216): 216ra177
- Beau-Faller M et al., Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014; 25(1): 126-131
- Yang JCH et al., Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015; 16(7): 830-838
- Udagawa H et al., Clinical outcome of non-small cell lung cancer with EGFR/HER2 exon 20 insertions identified in the LC-SCRUM Japan. J Thorac Oncol 2019; 14(10 suppl): S224 [abstract OA07.03]
- Hanna N et al., Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22(9): 1589-1597
- Kim ES et al., Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008; 372(9652): 1809-1818
- Neal J et al., Safety, PK, and preliminary antitumor activity of the oral EGFR/HER2 exon 20 inhibitor TAK-788 in NSCLC. J Thorac Oncol 2018; 13(10): S599
- Zhou C et al., Mobocertinib in NSCLC with EGFR exon 20 insertions: results from EXCLAIM and platinum pretreated patient populations. WCLC 2020, OA04.03
- Haura EB et al., JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). J Clin Oncol 37, 2019 (suppl; abstr 9009)
- Park K et al., Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (exon20ins)-mutated non-small cell lung cancer (NSCLC). J Clin Oncol 38: 2020 (suppl; abstr 9512)
- Sabari JK et al., Amivantamab in post-platinum EGFR exon 20 insertion mutant non-small cell lung cancer. WCLC 2020, OA04.04
- Girard N et al., Comparative clinical outcomes for patients with NSCLC harboring EGFR exon 20 insertion mutations and common EGFR mutations. WCLC 2020, MA04.07
- Bauml JM et al., Underdiagnosis of EGFR exon 20 insertion mutation variants: estimates from NGS-based real-world datasets. WCLCL 2020, FP07.12
- Tan CS et al., Third generation EGFR TKIs: current data and future directions. Mol Cancer 2018; 17(1): 29
- Scharpenseel H et al., EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci Rep 2019: 9(1): 7406
- Yi ES et al., High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol 1997: 10(2): 142-148
- Yu HA et al., Efficacy and safety of the novel HER3 directed antibody drug conjugate patritumab deruxtecan (HER3-DXd; US-1402) in EGFR-mutated NSCLC. WCLC 2020, OA03.04
- Wang P et al., Phase II study of the efficacy of the EGFR inhibitor mefatinib in patients with advanced EGFR-mutant NSCLC. WCLC 2020, P76.28
- Ramalingam SS et al., Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol 2018; 36(9): 841-849
- Schoenfeld AJ et al., Tumor analyses reveal squamous transformation and pff-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res 2020; 26(11): 2654-2663
- Cho BC et al., ORCHARD: A biomarker-directed phase 2 platform study in patients with advanced EGFRm NSCLC progressing on first-line osimertinib. WCLC 2020 P76.27
- Leonetti A et al., Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019; 121(9):725-737
- Rotow J et al., Combination osimertinib plus selpercatinib for EGFR-mutant non-small cell lung cancer with acquired RET fusions. WCLC 2020, FP14.07
- Fang W et al., Emergence of EGFR G724S After Progression on Osimertinib Responded to Afatinib Monotherapy. J Thorac Oncol 2020; 15(3): e36-e37
- Oztan A et al., Emergence of EGFR G724S mutation in EGFR-mutant lung adenocarcinoma post progression on osimertinib. Lung Cancer 2017; 111: 84-87
- Zhao H et al., Afatinib as a potential therapeutic option for non-small cell lung cancer patients with EGFR G724S. WCLC 2020, P76.38
- Ramalingam SS et al., Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382:41-50
- Lee CS et al., A comparison of sequential EGFR-TKI therapy versus first-line osimertinib in NSCLC: a multi-center, retrospective study. WCLC 2020, P76.09
© 2020 Springer-Verlag GmbH, Impressum