Updates on immunotherapy-based treatment of stage III/IV disease
IPSOS: atezolizumab in platinum-ineligible patients
At least 40 % of patients with NSCLC have poor performance status (ECOG PS ≥ 2) and/or are elderly with multiple comorbidities and poor tolerance of treatment [1]. They frequently receive single-agent chemotherapy or best supportive care as they are often deemed ineligible for first-line platinum-based regimens. Usually, they are excluded from clinical trials conducted in the first-line setting. This population represents an important, under-studied group with an unmet medical need.
Therefore, the global, open-label, randomized, controlled, phase III IPSOS study assessed atezolizumab 1,200 mg Q3W (n = 302) compared to single-agent chemotherapy (vinorelbine or gemcitabine; n = 151) in treatment-naïve patients with stage IIIB/IV NSCLC who were considered unsuitable for first-line platinum-doublet chemotherapy. This was due to either ECOG PS 2/3 or PS 0/1 combined with advanced age (≥ 70 years) and substantial comorbidities or other contraindications to platinum chemotherapy. IPSOS was conducted at 91 sites in 23 countries. Most patients had ECOG PS 2, and approximately one third was ≥ 80 years old. Overall survival (OS) constituted the primary endpoint.
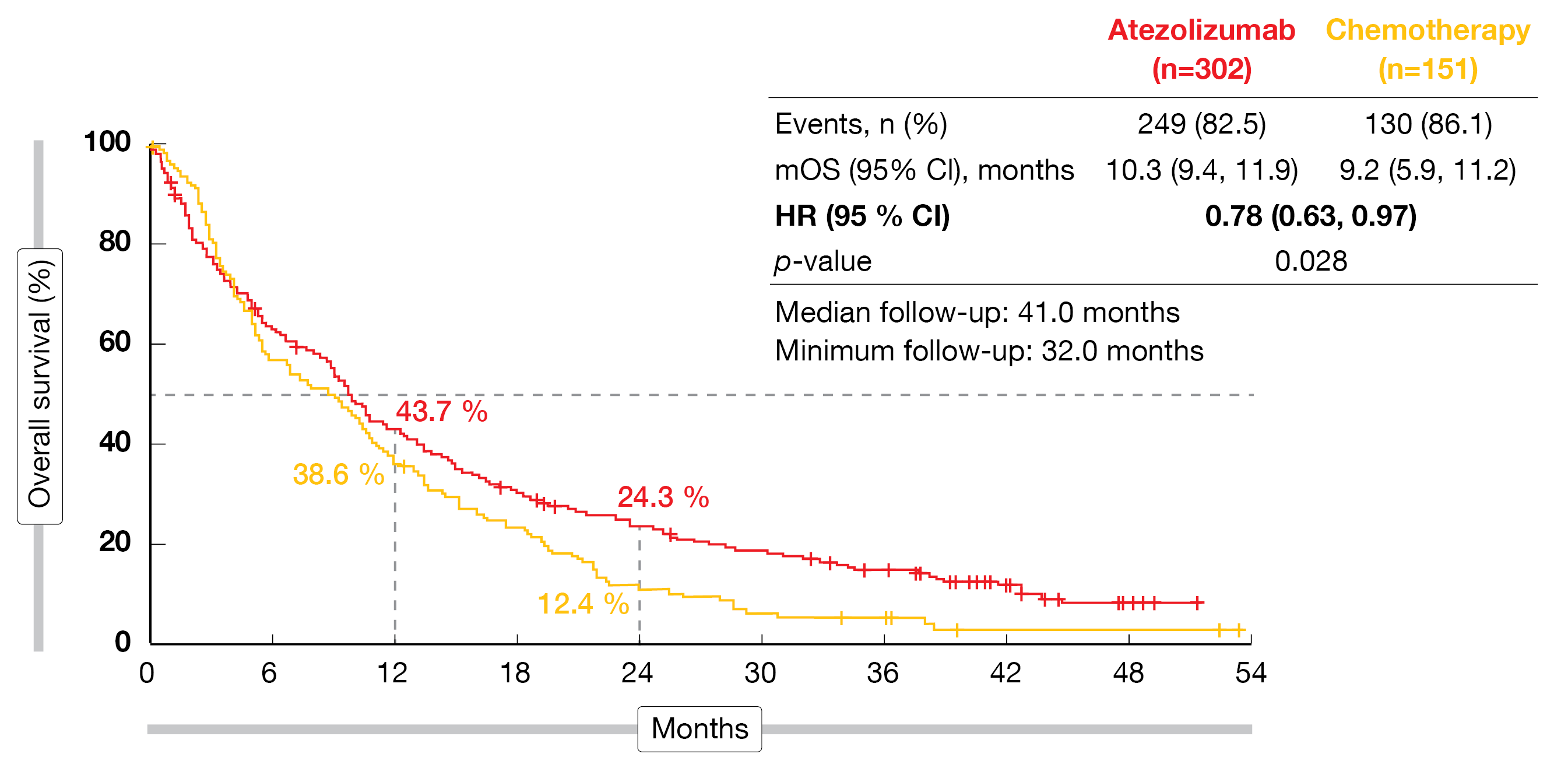
According to the analysis presented at ESMO 2022, first-line atezolizumab significantly improved OS over single-agent chemotherapy (10.3 vs. 9.2 months; HR, 0.78; p = 0.028; Figure 1) after a median follow-up of 41 months [2]. At 24 months, the proportion of patients alive was almost doubled in the experimental arm (24.3 % vs. 12.4 %). The superiority of the immune-based approach held true irrespective of PD-L1 status, histology, ECOG PS and other factors. Atezolizumab treatment gave rise to a higher objective response rate (ORR; 16.9 % vs. 7.9 %) and longer duration of response (14.0 vs. 7.8 months), while disease control was achieved in both arms to a comparable extent (57.3 % vs. 56.3 %). In the atezolizumab-treated arm, 4 patients (1.3 %) achieved complete responses (vs. 0 %). For progression-free survival (PFS), the analysis showed no difference (4.2 vs. 4.0 months; HR, 0.87), although there was a numerically higher risk reduction at 24 months (8.9 % vs. 1.6 %).
Figure 1: IPSOS: overall survival improvement with atezolizumab vs. single-agent chemotherapy in patients ineligible for platinum-based first-line chemotherapy
AEs and QoL outcomes
Treatment duration was nearly twice as long in the experimental arm (3.5 months for atezolizumab vs. 2.3 and 1.8 months for gemcitabine and vinorelbine, respectively). Nevertheless, fewer treatment-related grade 3/4 adverse events (AEs) occurred in the atezolizumab-treated population than in the chemotherapy group (16.3 % vs. 33.3 %). The same applied to treatment-related serious AEs (11.7 % vs. 15.6 %) and treatment-related grade 5 AEs (1.0 % vs. 2.7 %). AEs led to discontinuation in 13 % in both arms; the low rate indicated that the treatments were well tolerated. No new or unexpected AEs of special interest were observed with atezolizumab.
Prespecified patient-reported outcomes were assessed using the EORTC QLQ-C30 and QLQ-LC13 questionnaires. According to this, atezolizumab was associated with stabilization across all health-related quality of life functioning domains (i.e., role function, social function, cognitive function), while the chemotherapy arm showed some deterioration. Cough, appetite and chest pain improved in the experimental arm. On the other hand, patients in the control arm experienced deterioration regarding peripheral neuropathy, alopecia, and appetite. Time to confirmed deterioration was significantly longer with atezolizumab with respect to chest pain (HR, 0.51). As the authors noted in their summary, IPSOS is the first randomized study to demonstrate that first-line treatment with atezolizumab improves OS in this poor-prognosis population without driver mutations regardless of histology, PD-L1 status and ECOG PS while maintaining quality of life.
Dual PD-1/CTLA-4 inhibition: MEDI5752
MEDI5752 is a PD-1/CTLA-4 bispecific monoclonal antibody that fully binds PD-1 while preferentially binding CTLA-4 on PD-1–positive activated T cells, with the potential to improve efficacy and remain clinically tolerable [3]. A phase IB/II signal-finding study explored MEDI5752 in treatment-naïve patients with non-squamous NSCLC. The study contained a single-arm cohort testing 4 doses of MEDI5752 750 mg plus chemotherapy Q3W (n = 64) and a randomized part; here, the patients received either 4 doses of MEDI5752 1,500 mg plus chemotherapy Q3W (n = 20) or 4 doses of pembrolizumab 200 mg plus chemotherapy Q3W. Results from 91 patients were presented at ESMO 2022 by Ahn et al. [4]. The analyzed group included 41 patients in the randomized cohorts (20 and 21 in the experimental and control arms, respectively) and the first 50 patients in the single-arm cohort who had ≥ 8 weeks of follow-up.
MEDI5752 1,500 mg plus chemotherapy, as compared to pembrolizumab plus chemotherapy, induced numerical prolongation of OS (not reached vs. 16.5 months) and PFS (15.1 vs. 8.9 months). The ORRs were similar in the two arms (50.0 % vs. 47.6 %), although duration of response was doubled (20.5 vs. 9.9 months). In the PD-L1–negative group, 55.6 % vs. 30.0 % of patients responded.
Results obtained in the single-arm cohort that received MEDI5752 750 mg plus chemotherapy indicated encouraging efficacy, with 49 % of patients achieving ≥ 30 % reductions in target lesions and an ORR of 40.8 %. The PD-L1–negative cohort experienced ≥ 30 % reductions in target lesions in 55.6 % and responded in 44.4 %. Longer follow-up is required to assess response duration, PFS and OS in the single-agent group.
According to a translational analysis, MEDI5752 750 mg or 1,500 mg plus chemotherapy gave rise to higher peripheral CD4-positive T cell proliferation and clonal expansion than pembrolizumab plus chemotherapy. Tolerability of MEDI5752 750 mg plus chemotherapy was improved compared to MEDI5752 1,500 mg plus chemotherapy regarding rates of grade 3/4 treatment-related AEs (50 % vs. 80 %), ALT increases (all grades, 10 % vs. 55 %), and AEs leading to treatment discontinuation (20 % vs. 70 %). The authors concluded that MEDI5752 provides a potential option to improve upon standard of care in the first-line setting, especially in patients with PD-L1–negative NSCLC.
Nivo/ipi: what is the ideal treatment duration?
Limits for the duration of immunotherapy have been set arbitrarily without any firm evidence. At the same time, < 20 % of patients received 2 years of treatment in most of the randomized studies. In the phase III KEYNOTE-024, KEYNOTE-042, KEYNOTE-189, KEYNOTE-407 and CheckMate 227 trials, this proportion was 15.3 % [5-9]. CheckMate 227 that explored first-line nivolumab/ipilimumab showed that responses lasted for ≥ 2 years in 47 % of responders, while only 8-12 % of patients were still on treatment at 24 months. At 2 years, the PFS rate was 20 %.
The question arose whether the duration of immunotherapy treatment could be shortened without altering long-term survival in patients who have achieved disease control, particularly with a view to reducing financial toxicity and avoiding late immune-related AEs that are potentially threatening. In the randomized, phase III, non-inferiority DICIPLE trial, patients with stage IV NSCLC who had achieved disease control after 6 months of treatment with nivolumab/ipilimumab were randomized to either observation (stop & go arm) or continuation of the same regimen (continuation arm). Patients who progressed in the stop & go arm received nivolumab/ipilimumab again, whereas platinum-based doublet chemotherapy was recommended upon progression in the continuation arm. Zalcman et al. reported the first results of the DICIPLE study at ESMO 2022 [10]. The two arms included 35 and 36 patients, respectively.
The trial was interrupted as the combination treatment will not be registered or reimbursed in Europe. Therefore, statistically sound conclusions are impossible, although with more than 25 months of follow-up, no signal emerged of any detrimental effect of stopping immunotherapy at 6 months with respect to both PFS (35.2 vs. 20.8 months for stop & go and continuation, respectively) and OS (not reached in either arm) despite the low numbers. At 18 months, 93.7 % vs. 79.3 % of patients were alive (p = 0.33). The 12-month PFS rates were 81.2 % vs. 55.6 %.
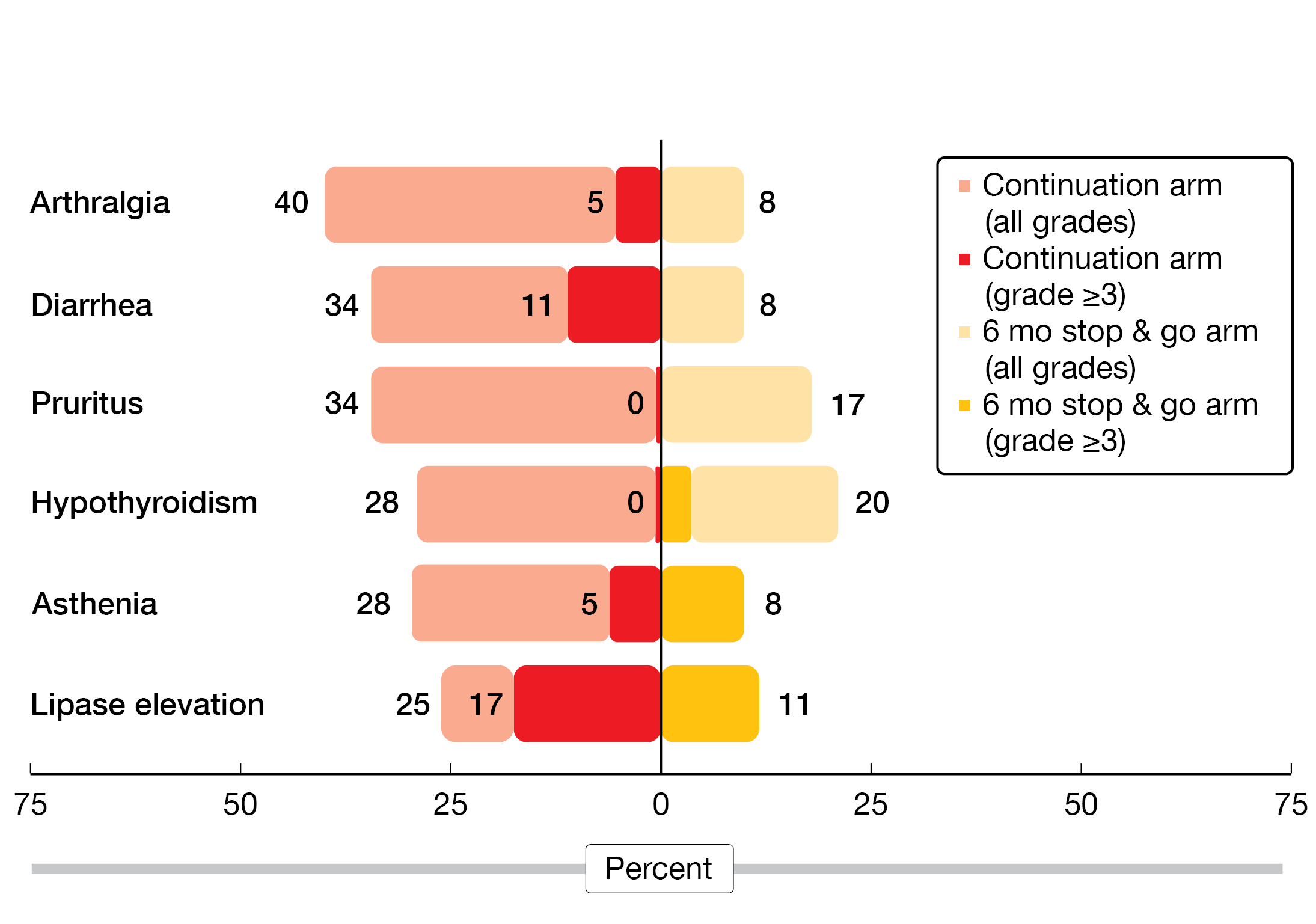
In addition, the stop & go strategy conferred numerically less grade 3/4 immune-related AEs by a factor of 10 (2.9 % vs. 28.6 %) and less serious grade 3/4 AEs (11.8 % vs. 25.7 %). All of the most frequent AEs showed diminished rates in the experimental arm (Figure 2). No patient died due to immune-related events. As the authors noted, these observations are only hypothesis-generating. Therefore, the phase II/III, randomized DIAL study has been launched with a similar design including induction treatment that contains platinum-based chemotherapy plus pembrolizumab (NCT05255302).
Figure 2: Continuation of nivolumab/ipilimumab after achievement of disease control vs. observation in stage IV NSCLC: most frequent adverse events post-randomization
Three-year update of EMPOWER-Lung 1
Single-agent treatment with the anti-PD-1 antibody cemiplimab was compared to 4–6 cycles of chemotherapy in the global, randomized, phase III EMPOWER-Lung 1 trial conducted in patients with untreated advanced NSCLC and PD-L1 ≥ 50 %. The PD-L1 ≥ 50 % population included more than 560 individuals randomized in a 1:1 manner. Overall, 138 sites in 24 countries participated in EMPOWER-Lung 1. After 30 months of follow-up, cemiplimab gave rise to significant prolongation of PFS (8.2 vs. 5.7 months; HR, 0.54; p < 0.0001) and OS (not reached vs. 14.2 months; HR, 0.57; p = 0.0002) [11].
The 3-year survival analysis presented at ESMO 2022 corroborated the initial results [12]. Cemiplimab, as compared to chemotherapy, led to further improvement of PFS (8.1 vs. 5.3 months; HR, 0.51; p = 0.0001) in the PD-L1 ≥ 50 % population. Meanwhile, median OS had been reached in the experimental arm, and the relative risk reduction was again 43 % (26.1 vs. 13.3 months; HR, 0.57; p = 0.0001). The OS benefit was obtained in spite of a 75 % crossover rate. Furthermore, checkpoint inhibition gave rise to higher ORR (46.5 % vs. 21.0 %; OR, 3.264; p < 0.0001), with complete remissions in 8.1 % vs. 2.1 % and considerably longer duration of response (23.6 vs. 5.9 months). The safety profile of the treatment after the extended follow-up was consistent with the known data.
Continued cemiplimab and chemo beyond progression
The study included optional continuation of cemiplimab plus 4 cycles of chemotherapy upon progression in the experimental arm; this was defined as “treatment period 2” following “treatment period 1” that had taken place between randomization and disease progression. Continuation beyond progression was tested in light of the absence of optimal choices in patients progressing on checkpoint inhibition in whom the switch to chemotherapy is the current standard of care. It was hypothesized that chemotherapy might facilitate response to cemiplimab by increasing cancer cell immunogenicity or selectively depleting immunosuppressive cells [13-16]. In EMPOWER-Lung 1, 64 patients received ≥ 1 cycle of chemotherapy in addition to continued cemiplimab after progression.
The comparison between treatment period 1 and treatment period 2 revealed similar response rates (ORR, 29.7 % and 31.3 %, respectively). Whereas none of these patient had achieved complete response in period 1, this applied to 3 patients in period 2 (4.7 %). Median PFS was similar, at 6.2 and 6.6 months, respectively. The time between randomization and death in periods 1 and 2 combined was 27.4 months, with 15.1 months being due to continued treatment in period 2. Compared to historical data for second-line treatment with chemotherapy (OS, 8.4 months), continued cemiplimab with the addition of chemotherapy appeared to be superior [17]. The safety profile in these 64 patients was consistent with the established profile.
Taken together, continued cemiplimab beyond progression with the addition of chemotherapy provided meaningful and durable benefits. As the authors pointed out, this is the first such report from a phase III study. The results underline the importance of cemiplimab as first-line chemotherapy-free treatment for patients with advanced/metastatic NSCLC and PD-L1 ≥ 50 %.
KEYNOTE-189: long-term outcomes
The randomized, phase III KEYNOTE-189 trial has established the first-line treatment standard of pembrolizumab plus platinum/pemetrexed in non-squamous NSCLC without EGFR/ALK alterations. This regimen significantly improved OS and PFS compared with placebo plus chemotherapy [18]. Garassino et al. reported the 5-year update of KEYNOTE-189 after a median follow-up of 64.6 months [19]. The effective crossover rate on or off study was 57.3 %.
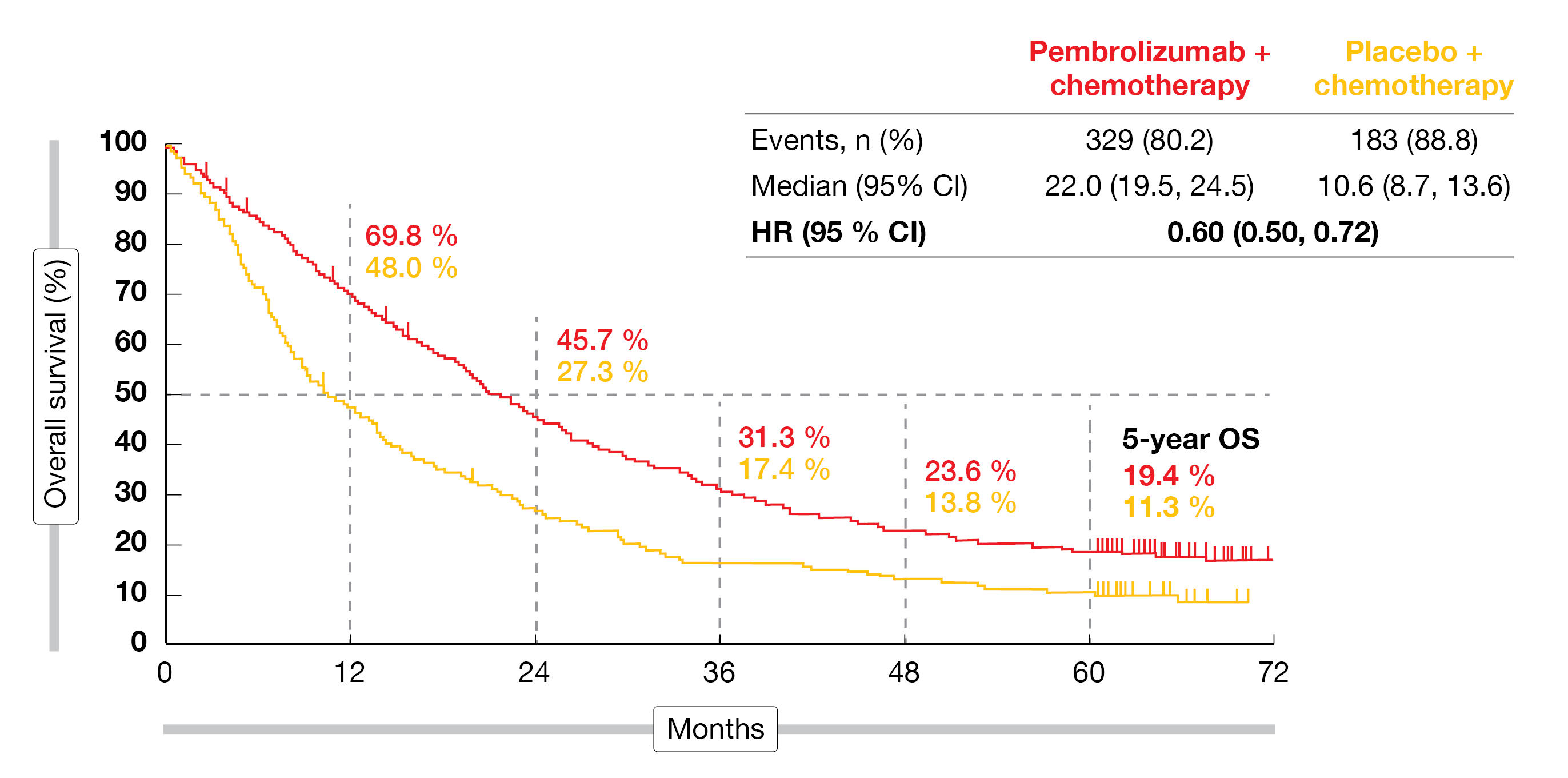
At 5 years, 19.4 % vs. 11.3 % of patients were alive, with median OS amounting to 22.0 vs. 10.6 months (HR, 0.60; Figure 3). The OS advantage was independent of PD-L1 expression and was also observed in the PD-L1–negative population. Likewise, superiority for PFS was maintained over the extended follow-up and despite the high crossover rate. Median PFS was 9.0 vs. 4.9 months, and the 5-year PFS rates were 7.5 % vs. 0.6 % (HR, 0.50). Again, the patients benefited from the addition of pembrolizumab irrespective of PD-L1 expression. In the total population, 48.3 % vs. 19.9 % of patients responded; the response rates were consistently higher in all PD-L1 subgroups.
New results have been obtained for 57 patients who completed 35 cycles of pembrolizumab treatment. In this cohort, the ORR was as high as 86.0 %, with 14.0 % and 71.9 % of patients achieving complete and partial responses, respectively. The median duration of response was 57.7 months. Three years after completion of the 35 pembrolizumab cycles, i.e., approximately 5 years from randomization, the OS rate was 71.9 %. Forty percent of patients were alive without disease progression or subsequent therapy. The long-term toxicity of the combined treatment proved manageable. Overall, these results continue to support first-line pembrolizumab plus platinum/pemetrexed as a standard of care in patients with metastatic non-squamous NSCLC without EGFR/ALK alterations.
Figure 3: Long-term overall survival in the KEYNOTE-189 trial
Findings in KEYNOTE-407 after 57 months
In the setting of untreated, metastatic NSCLC with squamous histology, the phase III KEYNOTE-407 trial assessed the combination of pembrolizumab plus carboplatin and paclitaxel/nab-paclitaxel. This regimen significantly improved OS and PFS compared to placebo and chemotherapy [8, 20]. After a median follow-up of 56.9 months, the efficacy and safety update presented at ESMO 2022 demonstrated sustained improvements regardless of the effective crossover rate of 50.9 % [21]. The 5-year OS rates were 18.4 % vs. 9.7 % for pembrolizumab plus chemotherapy vs. chemotherapy alone, and median OS was 17.2 vs. 11.6 months (HR, 0.71). In the groups with PD-L1 TPS ≥ 50 % and 1 %-49 %, the combined approach improved OS (HRs, 0.68 and 0.61, respectively), while the PD-L1–negative population did not benefit from the addition of pembrolizumab (HR, 0.83).
Moreover, the PFS analysis revealed an advantage in the experimental arm (8.0 vs. 5.1 months; HR, 0.62), and the 5-year PFS rates were 10.8 % and 3.5 %, respectively. Risk reductions occurred in all PD-L1 categories, with HRs of 0.48, 0.60 and 0.70 for PD-L1 TPS ≥ 50 %, 1 %-49 %, and < 1 %, respectively. In the ITT population, 62.2 % vs. 38.8 % of patients responded; median duration of response was 9.0 vs. 4.9 months. Superior responses resulted with pembrolizumab-based treatment in all PD-L1 groups.
Fifty-five patients completed 35 cycles of pembrolizumab treatment. This cohort achieved an ORR of 90.9 %. The rates for complete and partial responses were 16.4 % and 74.5 %, respectively, and the median duration of response had not been reached yet. At 3 years after the completion of 35 cycles, 69.5 % of patients were alive, and 43.6 % had not experienced disease progression or subsequent therapy. The toxicity was manageable and consistent with prior reports. These long-term data continue to support pembrolizumab plus chemotherapy as a standard-of-care first-line treatment option for patients with metastatic squamous NSCLC.
POSEIDON: OS by histology and mutational status
Dual immune checkpoint inhibition with the anti-PD-L1 antibody durvalumab and the CTLA-4 inhibitor tremelimumab in addition to chemotherapy has demonstrated significant benefits for PFS (HR, 0.72; p = 0.0003) and OS (HR, 0.77; p = 0.0030) compared to platinum-based chemotherapy alone in untreated patients with metastatic NSCLC included in the global, randomized, open-label, phase III POSEIDON study [22]. Durvalumab alone plus chemotherapy, as compared to chemotherapy, also showed significant PFS prolongation (HR, 0.74; p = 0.0009) and a trend for OS improvement (HR, 0.86; p = 0.0758). In the triple combination arm of the three-arm study, durvalumab, tremelimumab and chemotherapy Q3W for 4 cycles was followed by durvalumab Q4W until disease progression plus a fifth dose of tremelimumab in week 16. In the arm that received 4 cycles of durvalumab plus chemotherapy Q3W, maintenance therapy included durvalumab Q4W until progression.
After a median follow-up of approximately 4 years, Johnson et al. reported the OS update including analyses by histology and STK11, KEAP1 as well as KRAS mutational status [23]. The findings confirmed the durable long-term benefit of adding a limited course of tremelimumab to durvalumab and 4 cycles of chemotherapy, with median OS of 14.0 vs. 11.7 months in the chemotherapy-only arm and 48-month OS rates of 20.7 % vs. 8.3 % (HR, 0.75). For durvalumab plus chemotherapy vs. chemotherapy, this was 13.3 vs. 11.7 months (HR, 0.84). Data on serious AEs were collected after the final analysis for OS superiority; this analysis revealed no new safety signals.
Across the patient subgroups, the OS benefits were generally consistent with those in the ITT population. The addition of tremelimumab extended the sustained OS benefit to the cohort with PD-L1 < 1 %. Regarding histology, the survival advantage obtained with the triple combination was more pronounced in the group with non-squamous tumors; here, median OS was 17.2 vs. 13.1 months with chemotherapy, which translated into a 32 % risk reduction (HR, 0.68). At 48 months, 25.1 % vs. 10.0 % of patients were alive. The group with squamous histology showed median OS of 10.4 vs. 10.5 months, and the 48-month rates were 13.1 % vs. 5.5 % (HR, 0.83).
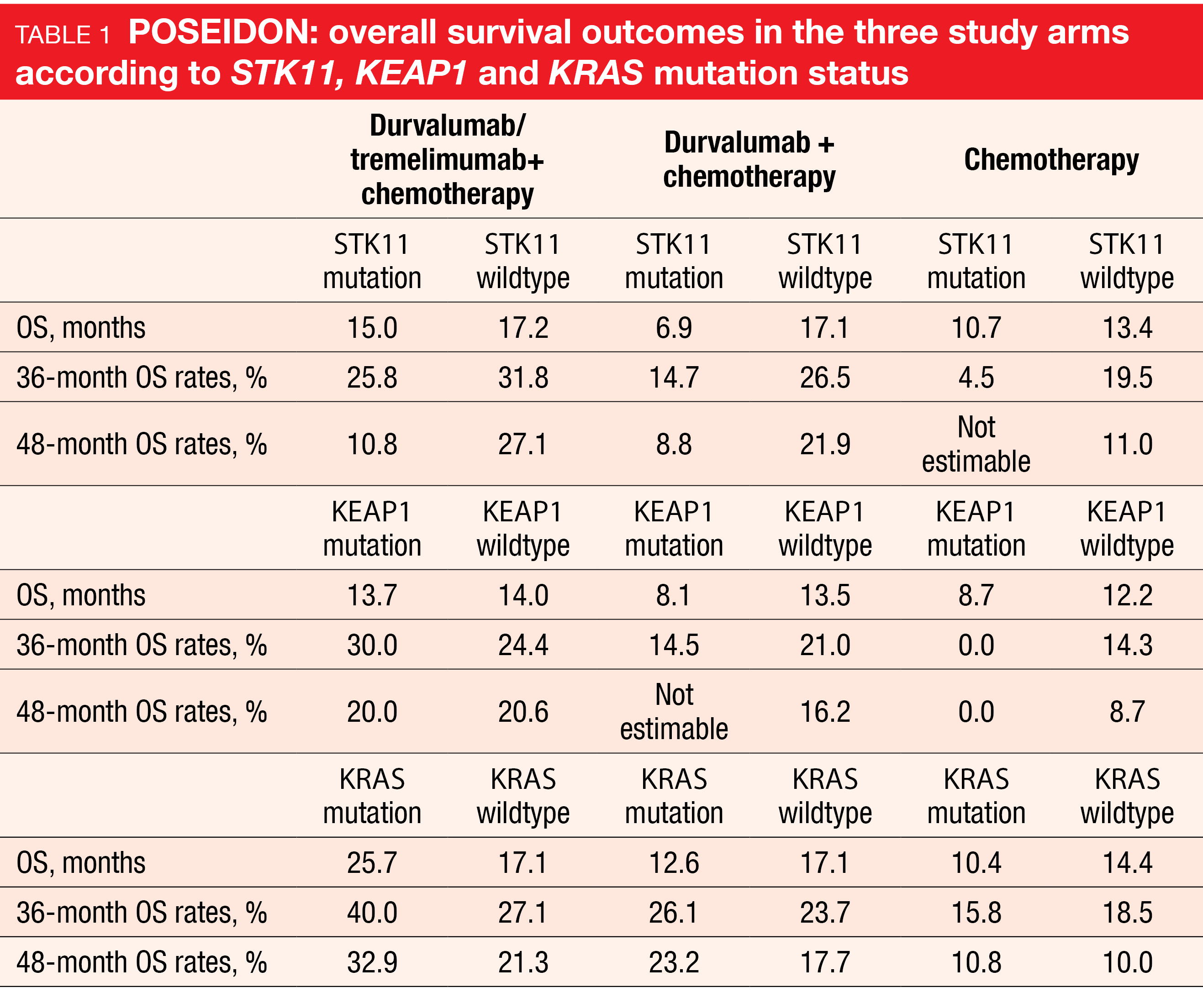
Patients with STK11, KEAP1 or KRAS mutations represent a difficult-to-treat population. Consistent with an earlier analysis [24], a trend for sustained OS benefits with the triple combination vs. chemotherapy was observed in the groups with STK11 (15.0 vs. 10.7 months; HR, 0.62), KEAP1 (13.7 vs. 8.7 months; HR, 0.43), and KRAS mutation (25.7 vs. 10.4 months; HR, 0.55; Table 1). In their conclusion, the authors emphasized that these findings support the use of durvalumab plus tremelimumab and chemotherapy as a first-line strategy for patients with metastatic NSCLC, including harder-to-treat patient subgroups.
Ociperlimab & tislelizumab: AdvanTIG
Combinations of TIGIT and PD-1 inhibitors have shown promising antitumor activity in early studies conducted in patients with NSCLC [25-27]. The ongoing phase IB AdvanTIG-105 study is assessing the anti-TIGIT antibody ociperlimab 900 mg Q3W together with the PD-1 inhibitor tislelizumab 200 mg Q2W and chemotherapy in patients with metastatic squamous NSCLC (Cohort 1; n = 40) and metastatic non-squamous NSCLC (Cohort 2; n = 42). At ESMO 2022, Yu et al. presented results from these two cohorts in the dose-expansion part of the study [28].
Ociperlimab and tislelizumab in addition to chemotherapy demonstrated antitumor activity independent of histology. ORR, which constituted the primary endpoint, was 57.5 % and 54.8 % in Cohorts 1 and 2, respectively. Disease control was achieved in 90.0 % and 90.5 %, respectively. Median duration of response had not been reached yet in either cohort. The recommended phase II dose showed a manageable safety profile. Treatment-emergent AEs primarily included anemia (42.9 %), decreased neutrophil counts (39.3 %), and decreased leukocyte counts (36.9 %). Grade ≥ 3 treatment-related AEs occurred in 48.8 %, and immune-mediated AEs were reported in 53.6 %. No treatment-related AEs led to death.
Ociperlimab plus tislelizumab is already being investigated in the phase II and III settings. The randomized, phase II AdvanTIG-205 study is comparing the anti-TIGIT/anti-PD-1 combination plus chemotherapy with tislelizumab plus chemotherapy in untreated patients with locally advanced or recurrent NSCLC not eligible for curative surgery [29]. Similarly, the randomized, phase III AdvanTIG-302 study has been designed as a first-line trial; here, ociperlimab plus tislelizumab is tested against pembrolizumab (NCT04746924).
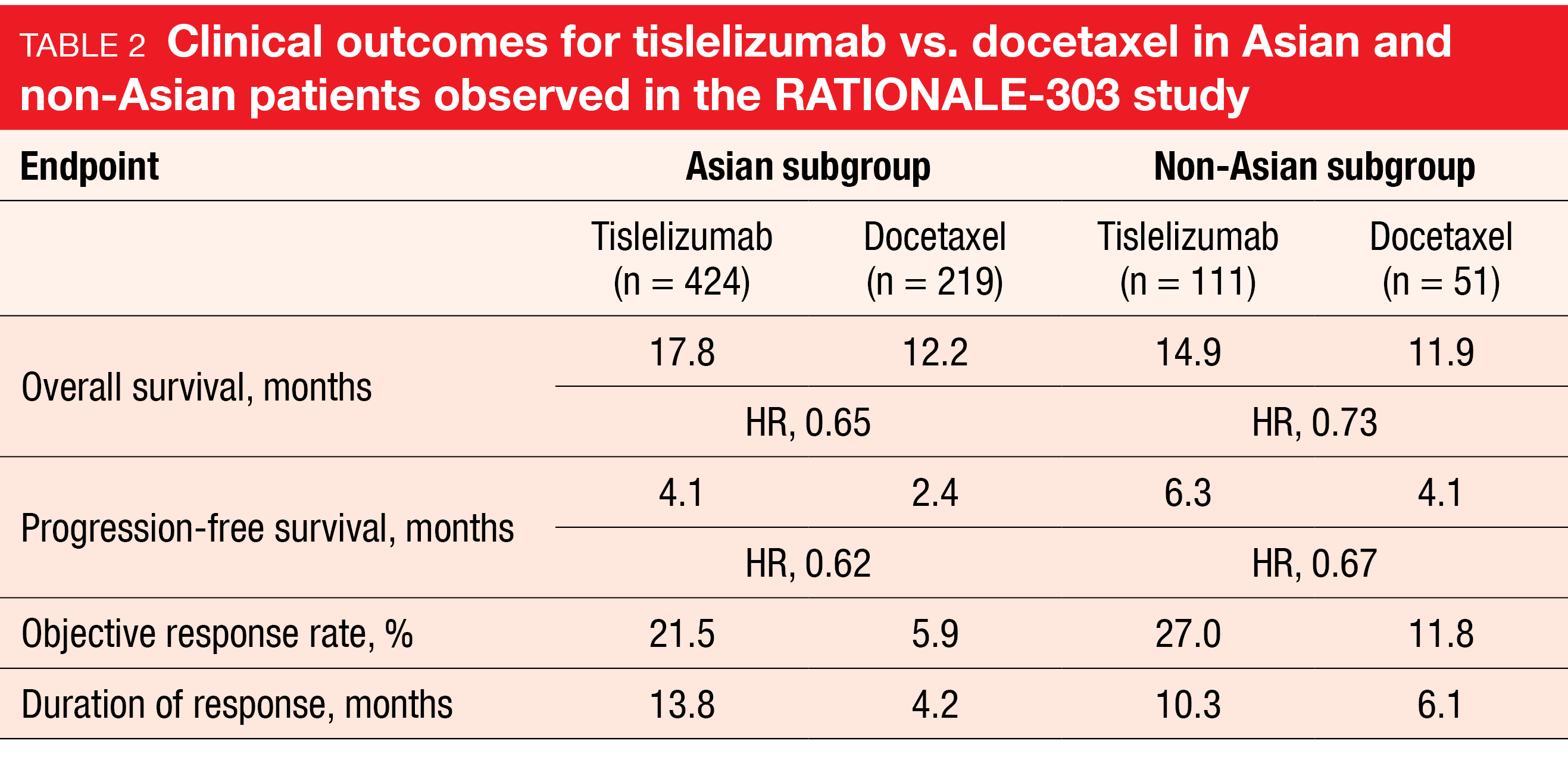
Asians vs. non-Asians in RATIONALE-303
In the phase III RATONALE-303 study, single-agent tislelizumab has demonstrated significant clinical benefit compared to docetaxel in patients with advanced squamous or non-squamous NSCLC who had progressed during or after ≥ 1 platinum-containing regimen but had received no more than two prior lines of systemic chemotherapy [30]. The analysis of the Asian (n = 643) and non-Asian (n = 162) subgroups presented at ESMO 2022 revealed consistent efficacy across the endpoints for the entire population (Table 2) [31]. Moreover, tislelizumab was generally well tolerated in both subgroups. Fewer grade ≥ 3 treatment-emergent AEs (TEAEs) were reported in the experimental arm vs. the control arm for both Asians (41.1 % vs. 75.2 %) and non-Asians (45.9 % vs. 72.9 %). TEAEs leading to treatment discontinuation occurred in 10.6 % vs. 12.4 % of Asian patients and in 17.1 % vs. 16.7 % of non-Asian patients.
As many patients do not respond to PD-(L)1-targeted monotherapy or develop resistance after initial response [32], the phase III SAFFRON-301 trial is currently exploring the combination of tislelizumab with the TKI sitravatinib [33]. Sitravatinib has both immunomodulatory and antitumor properties due to its targeting TAM family receptors (TYRO3, AXL, MER) and VEGFR2/KIT [34-36]. Patients with unresectable locally advanced or metastatic NSCLC after ≤ 2 lines of systemic therapy are treated with either tislelizumab plus sitravatinib or docetaxel. Prior treatment must include platinum-based chemotherapy and an anti-PD-(L)1 antibody. OS and PFS are defined as the primary endpoints.
REFERENCES
- Lilenbaum RC et al., Prevalence of poor performance status in lung cancer patients: implications for research. Thorac Oncol 2008; 3(2): 125-129
- Lee SM et al., IPSOS: results from a phase 3 study of first-line atezolizumab vs single-agent chemotherapy in patients with NSCLC not eligible for a platinum-containing regimen. ESMO 2022, abstract LBA11
- Dovedi SJ et al., Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1+ activated T cells. Cancer Discov 2021; 11(5): 1100-1117
- Ahn MJ et al., MEDI5752 or pembrolizumab plus carboplatin/pemetrexed in treatment-naïve non-small-cell lung cancer: a phase 1b/2 trial. ESMO 2022, abstract LBA56
- Reck et al., Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016; 375: 1823-1833
- Mok TSK et al., Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393(10183):1819-1830
- Ghandi L et al., Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med 2018; 378: 2078-2092
- Paz-Ares L et al., Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379(21):2040-2051
- Hellmann MD et al., Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med 2019; 381: 2020-2031
- Zalcman G et al., Nivolumab plus ipilimumab 6-month treatment versus continuation in patients with advanced non-small-cell lung cancer. ESMO 2022, abstract 972O
- Sezer A et al., Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50 %: a multicentre, open-label, global phase 3 randomised controlled trial. Lancet 2021; 397(10274): 592-604
- Özgüroglu M et al., Three-year survival outcome and continued cemiplimab beyond progression with the addition of chemotherapy for patients with advanced non-small cell lung cancer with PD-L1 ≥ 50 %: The EMPOWER-Lung 1 trial. ESMO 2022, abstract LBA54
- Galluzzi L et al., Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015; 28(6): 690-714
- Bloy N et al., Immunogenic stress and death of cancer cells: contribution of antigenicity vs adjuvanticity to immunosurveillance. Immunol Rev 2017; 280(1): 165-174
- Herbst RS et al., The biology and management of non-small cell lung cancer. Nature 2018; 553(7689): 446-454
- Galluzzi L et al., Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nature Rev Clin Oncol 2020; 17: 725-741
- Bersanelli et al., Chemotherapy in non-small cell lung cancer patients after prior immunotherapy: The multicenter retrospective CLARITY study. Lung Cancer 2020; 150: 123-131
- Gandhi L et al., Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 387(22): 2078-2092
- Garassino MC et al., KEYNOTE-189 5-year update: first-line pembrolizumab + pemetrexed and platinum vs. placebo + pemetrexed and platinum for metastatic nonsquamous NSCLC. ESMO 2022, abstract 973MO
- Paz-Ares L et al., A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 2020; 15(10): 1657-1669
- Novello S et al., 5-year update from KEYNOTE-407: pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer. ESMO 2022, abstract 974MO
- Johnson M et al., Durvalumab ± tremelimumab + chemotherapy as first-line treatment for mNSCLC: results from the phase 3 POSEIDON study. J Thorac Oncol 2021; 16(10_suppl): S844
- Johnson ML et al., Durvalumab ± tremelimumab + chemotherapy in 1L metastatic NSCLC: overall survival update form POSEIDON after median follow-up of approximately 4 years. ESMO 2022, LBA59
- Peters S et al., Association between KRAS/STK11/KEAP1 mutations and outcomes in POSEIDON: durvalumab ± tremelimumab + chemotherapy in mNSCLC. WCLC 2022, OA15.04
- Niu J et al., Safety and efficacy of vibostolimab, an anti-TIGIT antibody, plus pembrolizumab in patients with anti-PD-1/PD-L1-naïve NSCLC. Ann Oncol 2020; 31 (Suppl 4): S891-S892
- Rodriguez-Abreu D et al., Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab plus atezolizumab versus placebo plus atezo as first-line treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol 2020; 38 (15_suppl): 9503
- Ahn MJ et al., Vibostolimab, an anti-TIGIT antibody, as monotherapy and in combination with pembrolizumab in anti-PD-1/PD-L1-refractory NSCLC. Ann Oncol 2020; 31: S887
- Yu Y et al., AdvanTIG-105: phase 1b dose-expansion study of ociperlimab + tislelizumab with chemotherapy in patients with metastatic squamous and nonsquamous non-small cell lung cancer. ESMO 2022, abstract 1017P
- Zhu B et al., AdvanTIG-205: phase 2 trial of ociperlimab plus tislelizumab plus chemotherapy in first-line treatment of patients with locally advanced, unresectable, or metastatic non-small cell lung cancer. ESMO 2022, abstract 1194TiP
- Zhou C et al., Tislelizumab versus docetaxel in previously treated advanced non-small cell lung cancer: final analysis of RATIONALE-303. WCLC 2022, EP08.01-014
- Zhou C et al., Tislelizumab versus docetaxel as second- or third-line therapy in previously treated patients with advanced non-small cell lung cancer: Asian and non-Asian subgroup analysis of the RATIONALE-303 study. ESMO 2022, abstract 1031P
- Xia L et al., PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist 2019; 24(Suppl 1): S31-S41
- Zhou Q et al., SAFFRON-301: a phase 3 study of tislelizumab with sitravatinib versus chemotherapy in patients with locally advanced or metastatic non-small cell lung cancer previously treated with platinum-based chemotherapy and an anti-PD-1/PD-L1 antibody. ESMO 2022, abstract 1187TiP
- Du W et al., Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight 2018; 3(21): e124184
- Percent IJ et al., Phase III trial of sitravatinib plus nivolumab vs. docetaxel for treatment of NSCLC after platinum-based chemotherapy and immunotherapy (SAPPHIRE). J Clin Oncol 2020; 38(15_suppl): TPS9635
- Pircher A et al., Synergies of targeting tumor angiogenesis and immune checkpoints in non-small cell lung cancer and renal cell cancer: from basic concepts to clinical reality. Int J Mol Sci 2017; 18(11): 2291
© 2022 Springer-Verlag GmbH, Impressum
More posts
Preface – ESMO Lung Cancer 2022
Preface – ESMO Lung Cancer 2022 © ElainePerks2013 - Charles Swanton, MBBS, PhD, FRCP, F