Targeted approaches in the first and later lines: KRASG12C, EGFR, HER2 & angiogenesis
The first-in-class, oral, irreversible KRASG12C inhibitor sotorasib has given rise to durable clinical benefits in patients with advanced KRASG12C-mutated NSCLC in the phase II CodeBreak 100 trial, with an objective response rate (ORR) and median progression-free survival (PFS) of 37.1 % and 6.8 months, respectively [1]. Median overall survival (OS) was 12.5 months. CodeBreaK 200, the first phase III study for a KRASG12C inhibitor, compared sotorasib 960 mg/d (n = 171) with docetaxel 75 mg/m2 Q3W (n = 174) in patients with KRASG12C-mutated, locally advanced or metastatic NSCLC after ≥ 1 treatment including both platinum-based chemotherapy and checkpoint inhibition. Crossover from docetaxel to sotorasib was permitted after a protocol amendment.
Thirty-four percent of patients had a history of CNS involvement, while active brain metastases were not permitted. The majority had received ≥ 2 lines of therapy. PFS by blinded independent central review constituted the primary endpoint. Johnson et al. presented the primary analysis at the ESMO 2022 Congress [2].
Sotorasib as second-line standard
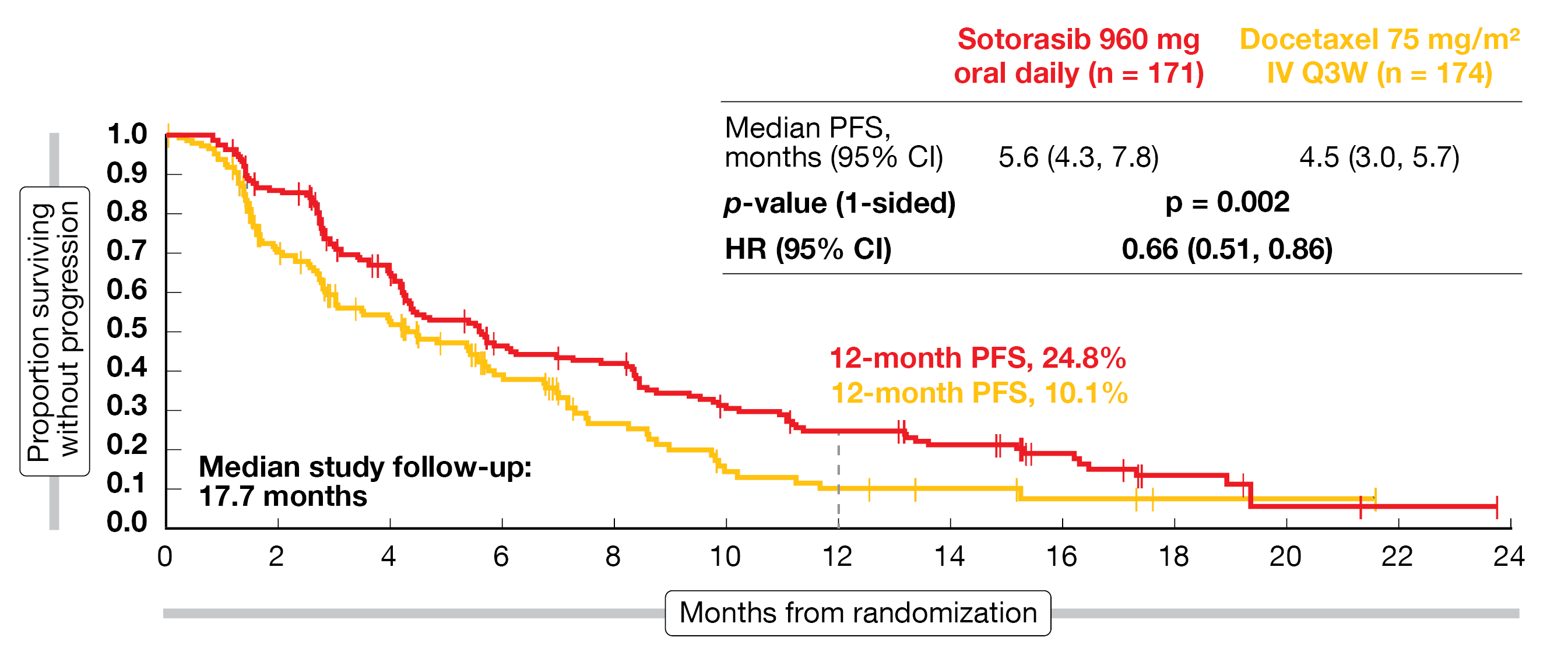
CodeBreaK 200 met its primary endpoint, with median PFS of 5.6 vs. 4.5 months (HR, 0.66; p = 0.002; Figure 1) after a follow-up of 17.7 months. The PFS rates at 12 months were more than double with sotorasib than with docetaxel (24.8 % vs. 10.1 %). All subgroups derived PFS benefits; an intriguing signal was the HR of 0.53 in patients with a history of CNS involvement, which hints at the potential of sotorasib to exert disease control in the CNS.
Likewise, a significantly higher proportion of patients in the experimental arm developed responses (28.1 % vs. 13.2 %; p < 0.001) and disease control (82.5 % vs. 60.3 %). Tumor shrinkage was achieved in 80.4 % and 62.8 % of patients, respectively. Sotorasib showed an association with both shorter time to response (1.4 vs. 2.8 months) and longer duration of response (DoR; 8.6 vs. 6.8 months). For OS, the analysis revealed no difference between the regimens (10.6 vs. 11.3 months; HR, 1.01; p = 0.53). However, the CodeBreaK 200 study was not powered to estimate OS, and 34 % of patients treated with docetaxel went on to receive subsequent KRASG12C inhibition, which included crossover.
Sotorasib was well tolerated. While the most common grade ≥ 3 treatment-related AEs (TRAEs) observed with the KRASG12C inhibitor included diarrhea and transaminase elevations, this was neutropenia, fatigue, and febrile neutropenia for docetaxel. Patients in the experimental arm experienced fewer grade ≥ 3 TRAEs (33.1 % vs. 40.4 %) and fewer serious TRAEs (10.7 % vs. 22.5 %) despite longer treatment (20 vs. 12 weeks).
The patient-reported outcomes analysis showed that the changes in global health status, physical functioning, and dyspnea from baseline to week 12 favored sotorasib. Compared with docetaxel, the targeted treatment significantly delayed the time to deterioration in global health status (HR, 0.69), physical functioning (HR, 0.69), and dyspnea as well as cough (HRs, 0.63 and 0.55, respectively). For chest pain, there was a trend in favor of sotorasib. Overall, these results support sotorasib as a new second-line standard option for patients with advanced KRASG12C-mutated NSCLC, and reinforce the importance of NGS testing for KRASG12C mutations.
Figure 1: Primary endpoint of CodeBreaK 200: superior progression-free survival with sotorasib
vs. docetaxel
APPLE: switch to osimertinib based on T790M monitoring
EGFR T790M mutations have been established as a strong predictive marker for the efficacy of the third-generation EGFR TKI osimertinib, with liquid biopsy representing a useful tool for genomic profiling. The randomized, phase II APPLE trial was designed to prospectively validate the benefit of dynamic treatment decisions based on longitudinal T790M assessment using liquid biopsy in patients with EGFR-TKI–naïve, advanced NSCLC harboring the common EGFR mutations deletion 19 and L858R mutation. First-line treatment was administered in 3 arms: the exploratory arm A received osimertinib until progression, while arms B (n = 52) and C (n = 51) were treated with gefitinib followed by osimertinib. Liquid biopsy analyses were conducted centrally once monthly in all arms, although they were only used for treatment decisions in arm B. In this arm, either detection of the T790M mutation or radiological progression by RECIST 1.1 prompted the switch from gefitinib to osimertinib, whereas in arm C, gefitinib was switched to osimertinib based on radiological progression alone. At ESMO 2022, Remon et al. presented the results obtained in arms B vs. C.
According to the results reported at ESMO 2022, 68 % and 77 % of patients in arms B and C, respectively, switched from gefitinib to osimertinib [3]. Serial monitoring of the T790M status identified 17 % of patients in arm B who had molecular progression before RECIST disease progression, thus giving rise to earlier treatment switches. Median time to molecular progression was almost 9 months. With respect to the PFS rates at 18 months on osimertinib treatment that were defined as the primary endpoint, the analysis showed a numerical advantage in arm B over arm C (67.2 % vs. 53.5 %; HR, 0.80; p = 0.22). Moreover, the patients experienced clinically meaningful PFS (22 vs. 20.2 months) and OS (not reached vs. 42.8 months). At 18 months, 87 % vs. 77 % of patients were alive.
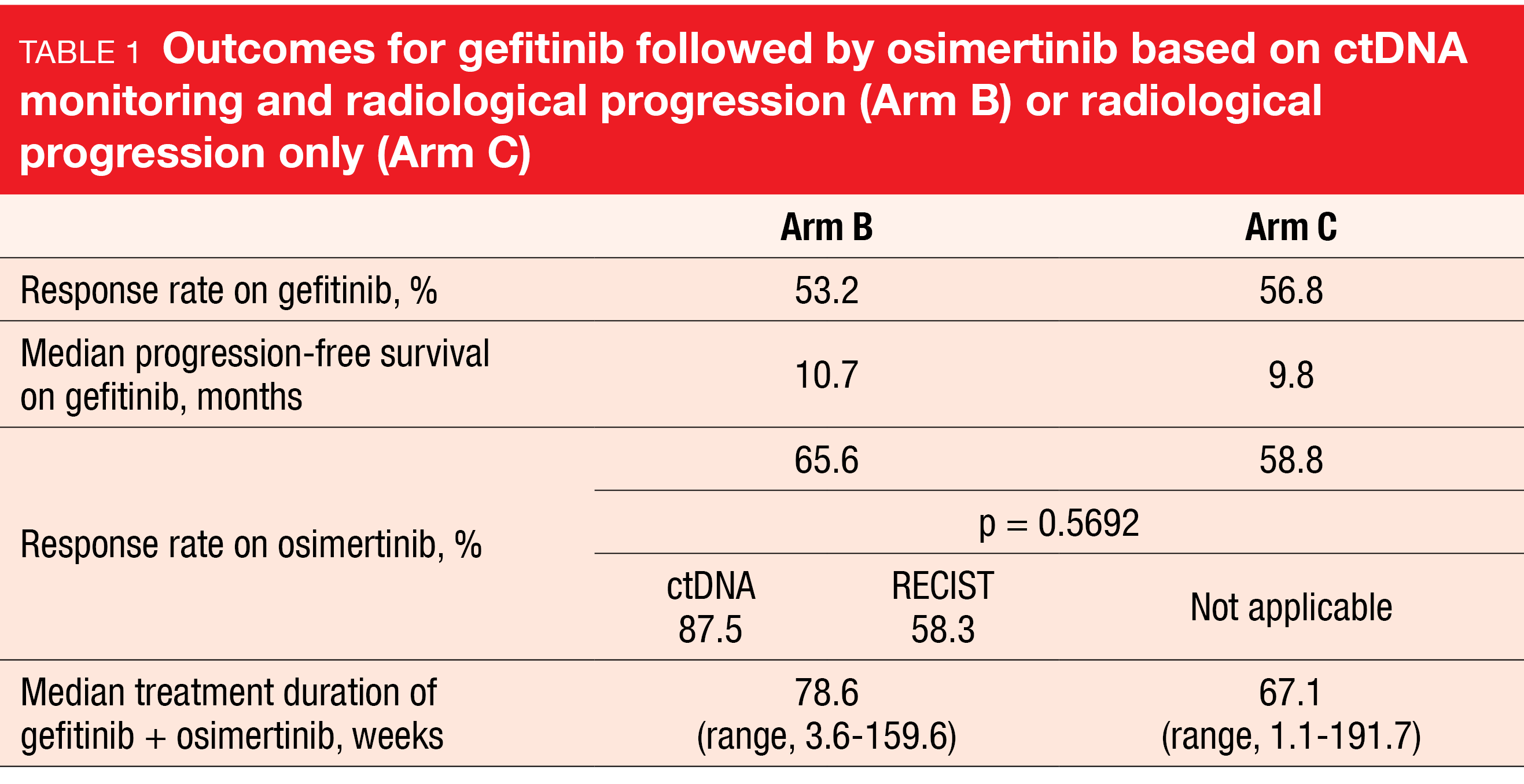
Response rates on osimertinib amounted to 65.6 % vs. 58.8 % in arms B vs. C, with longer median treatment duration in the experimental arm (Table 1). Within arm B, the response rate for patients who were switched based on ctDNA was higher than that for the group switched due to radiological progression. According to an exploratory analysis, ctDNA clearance of EGFR mutations on gefitinib treatment at weeks 4, 8 and 12 was an early predictor of longer PFS. As the authors pointed out, serial monitoring of the T790M status by ctDNA was shown to be feasible for informing treatment decisions in the setting of EGFR-mutant advanced NSCLC.
Molecular profiling of resistance mutations in ELIOS
In the first-line treatment setting of advanced EGFR-mutant NSCLC, osimertinib is a preferred option, although resistance eventually develops [4, 5]. Available tissue-based data on alterations at the time of first-line osimertinib resistance are limited. Therefore, the phase II ELIOS study characterized resistance mechanisms to first-line therapy with osimertinib in patients with locally advanced or metastatic NSCLC who had classical and atypical sensitizing EGFR mutations. Tissue biopsies were obtained prior to the start of treatment and at or after disease progression; paired sample analyses were performed by NGS and mass spectrometry. In addition, the patients underwent assessments according to RECIST 1.1 every 8 weeks. ctDNA was also collected longitudinally; these results will be presented at a future meeting. The proportion of patients with a given tumor genetic and proteomic marker at the time of disease progression constituted the primary endpoint. ELIOS is the first prospective study with a primary objective of comparing paired tumor tissue biopsies taken pre-treatment and after RECIST progression.
A total of 154 patients received ≥ 1 dose of osimertinib. Among the 119 patients who experienced disease progression, 75 provided biopsies pairs, while only 46 of these were evaluable (i.e., primary analysis set). According to the authors, this highlights the challenges of obtaining post-progression tissue biopsies and emphasizes the need for more comprehensive non-invasive testing methods. Paired proteomics data were available for 6 patients (i.e., proteomic data set). At ESMO 2022, Piotrowska et al. reported the first interim analysis of the ELIOS trial [6].
Alterations & increased proteomic expression
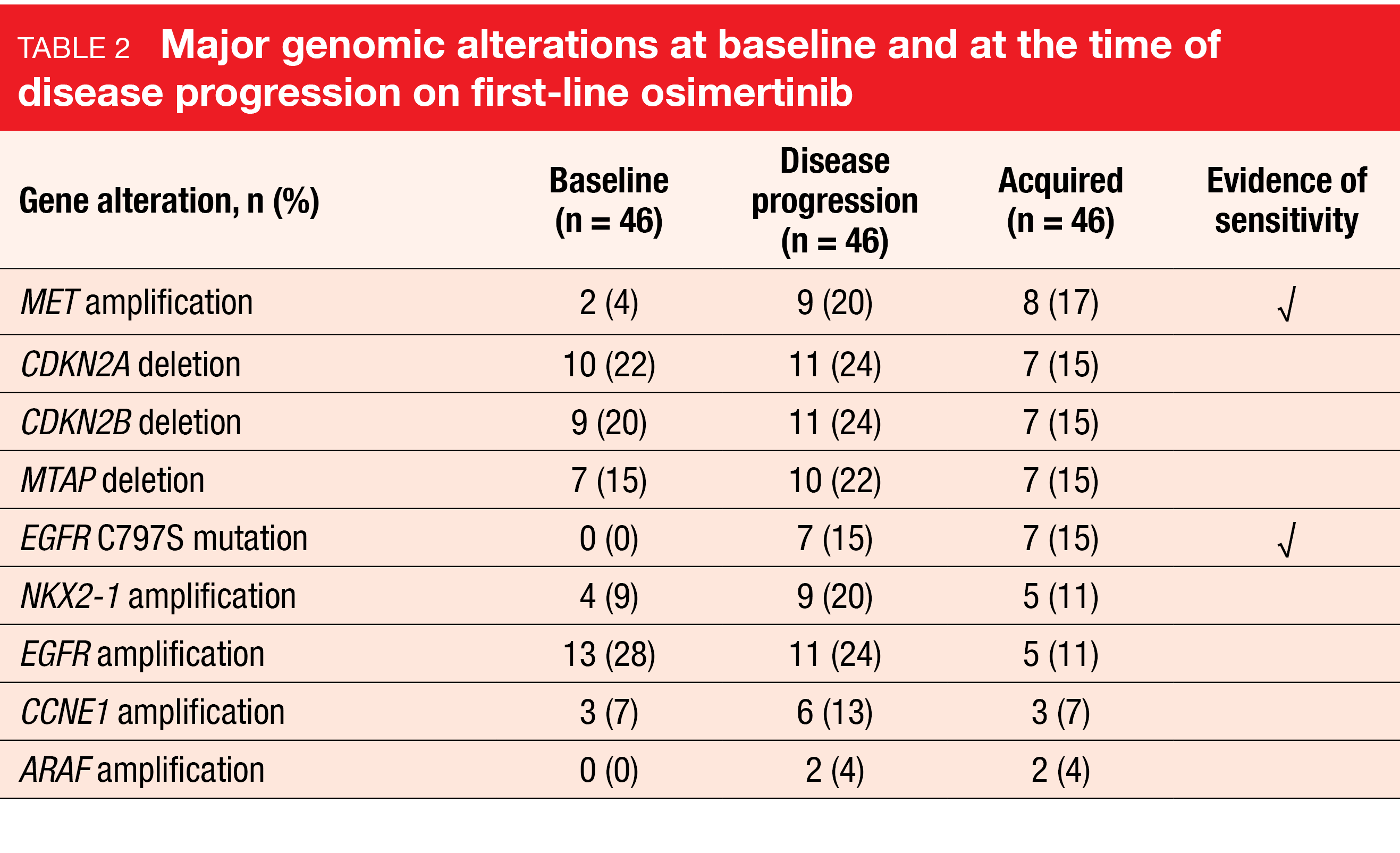
At baseline, TP53 mutations were the most common co-occurring mutations (74 %) in the primary analysis set, followed by EGFR amplifications (28 %) and CDKN2A loss (22 %). The prevalence of these alterations did not change significantly during the course of the study. With regard to the primary endpoint, Table 2 lists the frequencies of major alterations at baseline and at progression. Evidence of sensitivity to later-line targeted therapies is present for MET amplification, C797S mutation, and ALK fusion. Amplification of the NKX2-1 gene that codes for the TTF-1 protein was identified as a potential new resistance mechanism, although this requires further exploration and validation. Resistance mechanisms were well distributed between the different types of sensitizing mutations and were largely mutually exclusive.
The analysis of 15 selected markers from the proteomic data set revealed increased proteomic expression of the AXL and MET proteins by 67 % and 50 %, respectively, relative to baseline. No changes were observed for HER3 protein expression. Mass spectrometry confirmed higher AXL and MET expression at the time of progression, and upregulation of these markers in a subset of progressing tumors was consistent with prior data describing their role in acquired resistance to EGFR TKIs [7, 8]. Overall, the most prevalent acquired resistance mechanisms to osimertinib were MET amplification and overexpression, as well as EGFR C797S mutation. This was consistent with previous data [9-11]. Retrospective analyses on histologic transformation are ongoing, and molecular data are being collected for the remaining patients in the ELIOS trial.
INSIGHT 2: tepotinib/osimertinib
MET-amplification–mediated resistance against osimertinib is associated with a poor prognosis [12, 13]. In this setting with a high unmet need, combinations of the MET inhibitor tepotinib with the EGFR TKIs gefitinib and osimertinib have shown clinical activity in the INSIGHT study and the real-world setting, respectively [14, 15]. The global, open-label, phase II INSIGHT 2 trial is assessing the combination of tepotinib and osimertinib in patients with EGFR-mutated NSCLC and acquired resistance to first-line osimertinib in whom MET amplification has been detected by either central or local FISH testing of tissue biopsy (TBx), or central NGS testing of liquid biopsy (LBx). The second arm of the trial is receiving tepotinib monotherapy. Patients with stable, treated brain metastases are allowed to participate. MET amplification was defined as MET gene copy number (GCN) ≥ 5 and/or MET/CEP7 ratio ≥ 2 according to TBx FISH and/or MET GCN ≥ 2.3 (Archer®) according to LBx NGS. The primary endpoint is the ORR by independent review for tepotinib/osimertinib-treated patients with MET amplification centrally confirmed by TBx FISH.
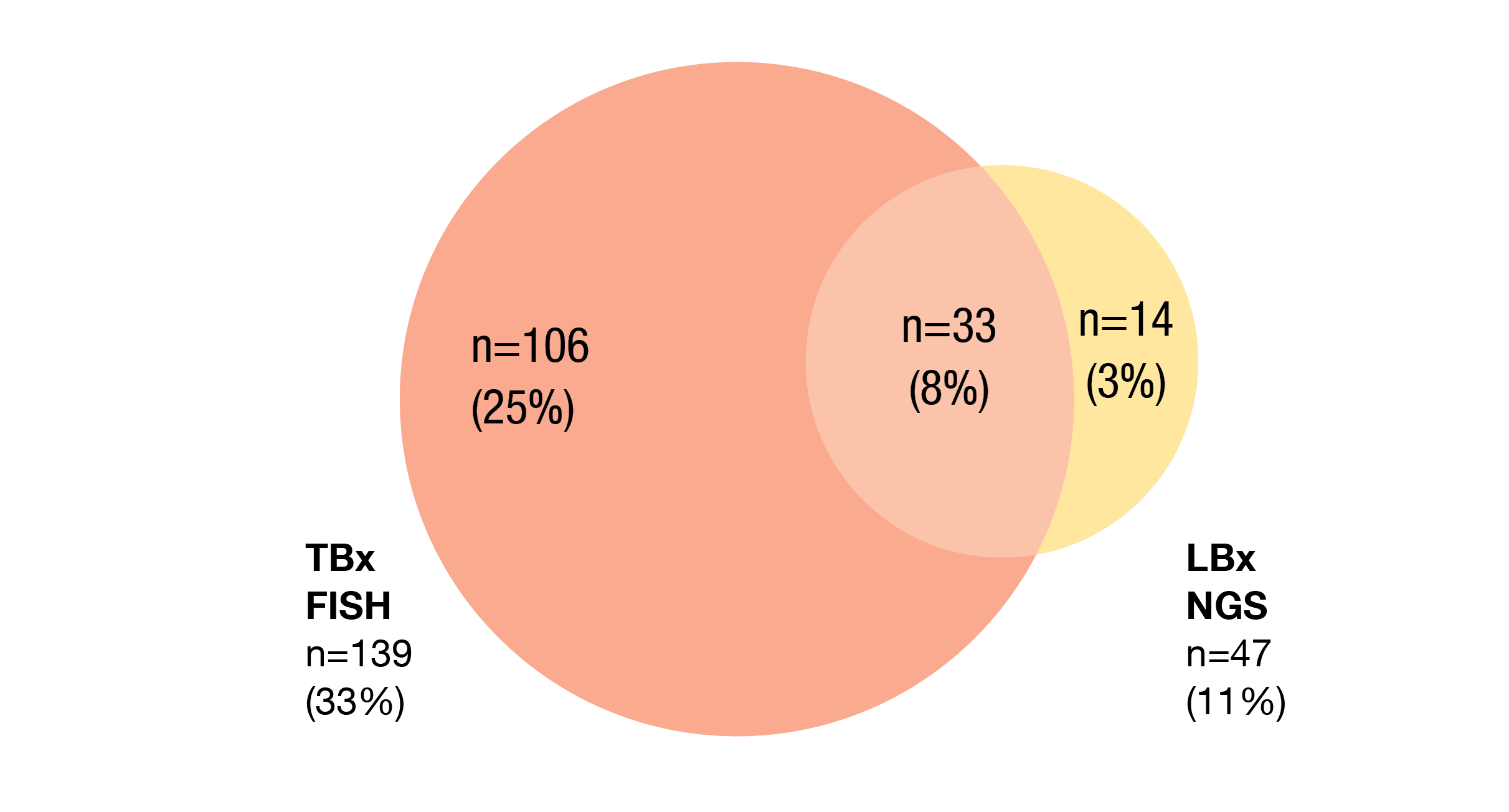
Among 425 pre-screened patients, MET amplification was detected in 153 patients (36 %). Most of them were identified by TBx FISH (Figure 2). The primary analysis of INSIGHT 2 will be reported when all patients have ≥ 9 months of follow-up. At ESMO 2022, Mazières et al. presented initial results [16]. In the combination arm, 48 and 22 patients had a median follow-up of ≥ 3 and ≥ 9 months, respectively. Twelve patients in the tepotinib monotherapy arm had been followed for ≥ 6 months.
Figure 2: Proportions of patients with MET amplification detected by tissue-based (TBx) FISH and liquid-biopsy–based (LBx) NGS
Superiority of the combination approach
In the tepotinib/osimertinib-treated group with centrally confirmed MET amplification according to TBx FISH and ≥ 9 months of follow-up, the confirmed ORR by independent review was 54.5 %. A comparable rate of 45.8 % was observed for patients with ≥ 3 months of follow-up. For patients whose MET amplification status was based on LBx NGS, the ORRs were 50.0% and 56.5 % after a follow-up of ≥ 9 months and ≥ 3 months, respectively. Similar ORRs were reported according to MET amplification GCN (51.9 % and 40.0 % for ≥ 10 GCN and 5 to ≤ 10 GCN, respectively). Single-agent tepotinib, on the other hand, gave rise to an ORR of only 8.3 %.
The vast majority of patients experienced tumor shrinkage with tepotinib and osimertinib. Patients with brain metastases (n = 22) responded as well. Responses mostly occurred within 6 weeks and were observed regardless of the GCN cutoff. Median DoR had not been reached at the time of the analysis. The safety profile of the combination was consistent with the known profiles of tepotinib and osimertinib. AEs led to dose reductions in 18.2 %, and 6.8 % of patients discontinued treatment, primarily due to AEs.
In their summary, the authors noted that tepotinib plus osimertinib showed promising activity in patients with EGFR-mutant NSCLC and MET amplifications centrally confirmed by TBx FISH who progressed on first-line osimertinib, thus providing a potential chemotherapy-sparing targeted option in this setting. In addition, FISH MET GCN of ≥ 5 and/or a MET/CEP7 ratio of ≥ 2 in TBx samples appeared to define a population that derives clinical benefit from the combination.
T-DXd: DESTINY-Lung02
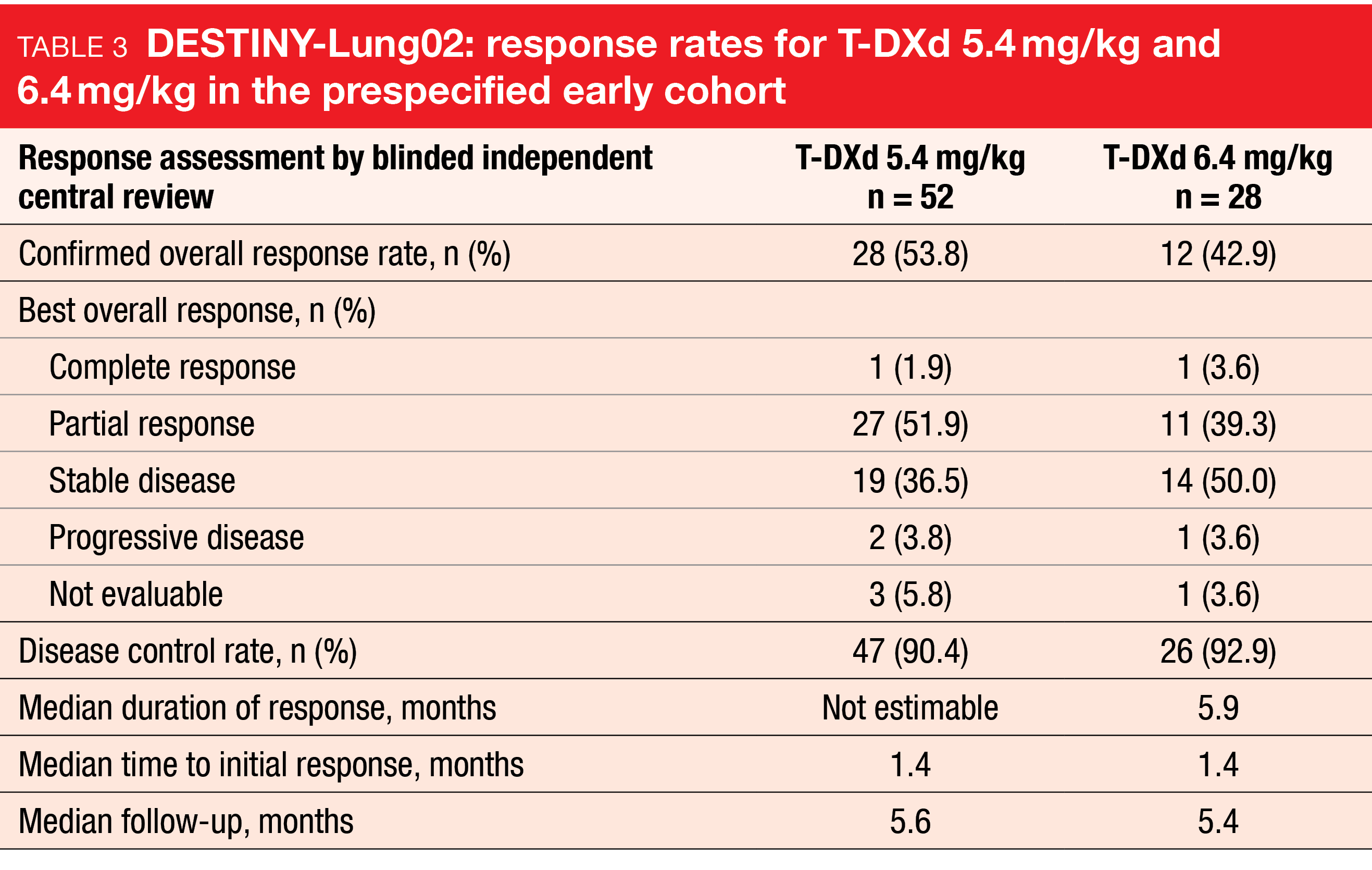
The HER2-directed antibody-drug conjugate trastuzumab deruxtecan (T-DXd) has shown strong and durable clinical activity in patients with pretreated HER2-mutant NSCLC in the DESTINY-Lung01 study [17]. As these results warranted evaluation of the benefit/risk profile of T-DXd 5.4 mg/kg and further assessment of T-DXd 6.4 mg/kg, the randomized, phase II DESTINY-Lung02 trial is investigating these two doses in patients with metastatic HER2-mutant NSCLC after ≥ 1 treatment line including platinum-based chemotherapy. DESTINY-Lung02 is not powered to statistically compare the two arms. The confirmed ORR by blinded independent review constitutes the primary outcome. Findings from the prespecified early cohort (i.e., patients randomized ≥ 4.5 months before the interim analysis data cutoff) were presented at ESMO 2022 [18]. In this group, 52 and 28 patients received T-DXd 5.4 mg/kg and T-DXd 6.4 mg/kg, respectively. The safety analysis set included the total study population comprising 101 and 50 patients in the two arms.
T-DXd 5.4 mg/kg induced clinically meaningful responses, with an ORR of 53.8 % and a disease control rate of 90.4 %. As the median DoR had not been reached yet, an additional 90-day follow-up response analysis was conducted that yielded a median DoR of 8.7 months. The ORR had risen to 57.7 % after the longer follow-up, thus continuing to demonstrate strong and clinically meaningful antitumor activity. For T-DXd 6.4 mg/kg, the ORR and the disease control rate were 42.9 % and 92.9 %, respectively, and median DoR was 5.9 months (Table 3). Mean tumor size reductions amounted to -38.6 % and -34.6 % for the 5.4 mg/kg and 6.4 mg/kg doses, respectively.
The safety profile at both doses was consistent with the established safety profile of T-DXd, although the 5.4 kg/mg dose conferred lower rates of drug-related treatment-emergent AEs that were grade ≥ 3 (31.7 % vs. 58.0 %) or associated with drug discontinuation (7.9 % vs. 16.0 %), dose reduction (9.9 % vs. 26.0 %), drug interruption (13.9 % vs. 30.0 %), and death (1.0 % vs. 2.0 %). Any-grade adjudicated drug-related interstitial lung disease rates favored the lower T-DXd dose (5.9 % vs. 14.0 %). Most cases were rated as grade 1 or 2, and 50 % had resolved at the time of the analysis. As the authors noted, the entire evidence and the compelling positive benefit-risk balance support T-DXd 5.4 mg/kg as a new standard of care in patients with pretreated HER2-mutant NSCLC. Interstitial lung disease/pneumonitis remains an important identified risk, with effective early detection and management being critical in reducing the severity of this complication.
Nintedanib/docetaxel after chemo-IO
Tumor angiogenesis is involved in the development of resistance to immune checkpoint inhibitors by promoting an immunosuppressive tumor microenvironment [19]. Angiogenesis-targeted agents are hypothesized to support the development of a pro-immunogenic microenvironment by the so-called angio-immunogenic switch [19, 20], thus reversing acquired resistance to immunotherapy. Cohort C of the prospective, non-interventional VARGADO study assessed the second-line use of the angiokinase inhibitor nintedanib plus docetaxel in patients with advanced adenocarcinoma of the lung who had progressed on first-line chemotherapy plus immunotherapy in routine clinical practice at centers in Germany. The analysis reported at ESMO examined treatment outcomes according to patient response to first-line therapy and ECOG performance status (PS) at baseline [21]. The best responses to chemotherapy/immunotherapy had been partial response (PR) and stable disease (SD) in 37.8 % and 25.9 % of patients, respectively, with 34.1 % progressing.
In 176 patients, the ORR associated with the combination was 35.0 %, and disease control resulted in 68.3 %. Median PFS and OS were 4.8 and 8.1 months, respectively. Among response-evaluable individuals, the ORR with nintedanib/docetaxel was improved in patients who had experienced PR or SD as best response to their first-line therapy (40.0 %) versus those who had developed disease progression (28.6 %). The same applied to the disease control rate (73.3 % vs. 62.9 %). Therefore, the best response to first-line treatment might be a surrogate marker for the clinical benefit of second-line nintedanib/docetaxel.
Another potential surrogate marker is the ECOG PS, as especially patients with PS 0-1 showed an association between the OS benefit observed in the second line and PR as best response to first-line therapy. In this group, median OS was 11.2 months. No clear differences emerged regarding PFS and best response to first-line treatment. The findings warrant further evaluation of the effect of these parameters on second-line treatment outcomes after chemoimmunotherapy. Taken together, these data support second-line nintedanib plus docetaxel as an effective treatment option following first-line checkpoint inhibitor combination therapy.
REFERENCES
- Skoulidis F et al., Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med 2021; 384: 2371-2381
- Johnson ML et al., Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12 mutation: CodeBreaK 200 phase 3 study. ESMO 2022, abstract LBA10
- Remon J et al., Osimertinib treatment based on plasma T790M monitoring in patients with EGFR-mutant advanced non-small cell lung cancer: EORTC Lung Cancer Group 1613 APPLE phase II randomized clinical trial. ESMO 2022, abstract LBA51
- Planchard D et al., Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29(Suppl 4): iv192-iv237
- Leonetti A et al., Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019; 121(9): 725-737
- Piotrowska Z et al., ELIOS: a multicentre, molecular profiling study of patients with EGFRm advanced NSCLC treated with first-line osimertinib. ESMO 2022, abstract LBA53
- Wang F et al., Blockade of AXL activation overcomes acquired resistance to EGFR tyrosine kinase inhibition in non-small cell lung cancer. Transl Cancer Res 2019; 8(6): 2425-2438
- Piotrowska Z et al., MET amplification as a resistance mechanism to osimertinib. J Clin Oncol 35, 2017 (suppl; abstr 9020)
- Hartmaier RJ et al., Tumor genomics in patients with advanced epidermal growth factor receptor mutant non-small cell lung cancer whose disease has progressed on first-line osimertinib therapy in the phase II ORCHARD study. Cancer Res 2022; 82(Suppl 12): LB078
- Ramalingam SS et al., Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. ESMO Congress 2018, abstract 5005
- Ahn MJ et al., MET biomarker-based preliminary efficacy analysis in SAVANNAH: savolitinib+osimertinib in EGFRm NSCLC post-osimertinib. WCLC 2022, EP08.02-140
- Wang Y et al., Clinical analysis by next-generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer 2018; 118: 105-110
- Koulouris A et al., Resistance to TKIs in EGFR-mutated non-small cell lung cancer: from mechanisms to new therapeutic strategies. Cancers 2022; 14(14): 3337
- Wu YL et al., Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med 2020; 8(11): 1132-1143
- Le X et al., Tepotinib with an EGFR-tyrosine kinase inhibitor in patients with EGFR-mutant MET-amplified NSCLC: A Case series. WCLC 2022, EP08.02-162
- Mazières J et al., Tepotinib + osimertinib for EGFRm NSCLC with MET amplification after progression on first-line osimertinib: initial results from the INSIGHT 2 study. ESMO 2022, abstract LBA52
- Nakada T et al., The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo) 2019; 67(3): 173-185
- Goto K et al., Trastuzumab deruxtecan in patients with HER2 mutant metastatic non-small-cell lung cancer: interim results from the phase 2 DESTINY-Lung02 trial. ESMO 2022, abstract LBA55
- Popat S et al., Anti-angiogenic agents in the age of resistance to immune checkpoint inhibitors: Do they have a role in non-oncogene-addicted non-small cell lung cancer? Lung Cancer 2020; 144: 76-84
- Fukumura D et al., Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018; 15(5): 325-340
- Grohé C et al., Effect of best response to first-line treatment on outcomes with second-line nintedanib + docetaxel for patients with lung adenocarcinoma after first-line immune checkpoint inhibitor/chemotherapy combination therapy. ESMO 2022, abstract 1143P
© 2022 Springer-Verlag GmbH, Impressum
More posts
Preface – ESMO Lung Cancer 2022
Preface – ESMO Lung Cancer 2022 © ElainePerks2013 - Charles Swanton, MBBS, PhD, FRCP, F